With a Novel Therapy that May Reverse the Course of Parkinson’s Disease, This Emerging Pharma Company is Developing Novel Therapies for Neurodegenerative Disease.
Inhibikase Therapeutics, Inc. (Nasdaq: IKT) is Creating Disruptive Solutions for Neurodegenerative Diseases Including Parkinson’s Disease!

Download The Corporate Presentation
Ask anyone whose life has been affected by Parkinson's disease and they will tell you just how important development for effective treatments is.
Every six minutes, someone in the U.S. is diagnosed with the disease. A new study has also estimated that nearly 90,000 people in the U.S. are diagnosed with Parkinson’s disease (PD) each year. This represents a steep 50% increase from the previous estimate of 60,000 diagnosed per year (see Parkinson’s Disease Foundation Decisions Resources 2016, Lewin Report in the Economic Burden and Future Impact of Parkinson’s disease, 2019).
The worst part is that there has been virtually no progress in treating it over the last 50 years!
It comes as no surprise that the biotech space is filled with companies who are working on potentially life-altering treatments for Parkinson’s.
One company developing a new approach to treatment of Parkinson’s disease is Inhibikase Therapeutics, Inc. (Nasdaq: IKT), a clinical-stage pharmaceutical company innovating small-molecule kinase inhibitor therapeutics.

These novel therapeutics are aimed to treat Parkinson's Disease and other neurodegenerative diseases.
Reasons Why IKT is Standing Out?
- Despite the market volatility in 2023, shares closed through February 5th, 2024 up 214% from its all time low of $0.81 on October 9, 2023.
- The company has a cash/cash equivalent runway into 4Q24.
- An experienced management team that has aided the company in making great strides across its multi-therapeutic pipeline.
- A robust patent portfolio with protection to 2033 (oncology) and 2036 (neurogenderation)
- The company is advancing its 201 trial of evaluating risvodetinib in untreated Parkinson’s disease and began actively enrolling patients in the second quarter of 2023.
- Results from the 201 trial are anticipated in the second half of 2024, depending on the last patient enrollment
- The company’s approach to Multiple System Atrophy, or MSA, an aggressive form of Parkinson’s disease, has seen notable pre-clinical success in 2023.
- The company has opened an IND for direct entry into a Phase 2 clinical trial and has received Orphan Drug Designation for risvodetinib as a treatment for MSA from the FDA.
- The company plans to submit complementary regulatory documents for orphan designation for risvodetinib to treat MSA to European Union authorities in 2024.
- The company’s cancer therapeutics program has made significant progress throughout 2023.
- IkT-001Pro, the company’s prodrug of imatinib, was evaluated in 66 healthy subjects, ages 18 to 55, to measure bioequivalence to 400 mg or 600 mg imatinib mesylate. With these studies complete, the company had a pre-NDA teleconference with the FDA in January 2024.
- The company is also evaluating the market potential of IkT-001Pro in non-oncology indications to which imatinib has already been shown to have meaningful benefits.
2023 was a year of growth for IKT’s clinical and chemistry teams who advanced its clinical programs across multiple therapeutic areas.
2024 is anticipated to be a year of advancements scientifically and medically, where IKT believes its efforts could show promise for the treatment of Parkinson’s disease and other diseases of the Abelson Tyrosine Kinases.
Greetings Investors,
A key challenge with investing in pharmaceutical companies is figuring out which disease areas might offer the best promise. One area that's ripe for investment is treatments for neurodegenerative diseases like Parkinson’s.
What is Parkinson’s disease?
Parkinson's is a devastating neurological disease that is expected to rise exponentially in the next decade.
Michael J. Fox has been one of the most famous people associated with the disease. When the Emmy and Golden Globe Award-winning actor was diagnosed with Parkinson's disease at age 29, his clearest symptom was a small one — his pinky finger was twitching.
Symptoms include tremors in the hands, although patients can also have tremors in the legs, jaw, or head. Other symptoms include muscle stiffness, slow movements, impaired balance, and impaired coordination and balance. In addition to those movement-related symptoms, patients may also experience emotional changes like depression, skin problems, constipation or urinary problems, or difficulties chewing, swallowing, and speaking.
These symptoms have been traced to rapidly declining dopamine levels. Dopamine is the chemical messenger responsible for transmitting messages between the nerves that control muscle movements and those related to the reward and pleasure centers in the brain.
The Market
The global Parkinson's disease treatment market was estimated to be worth $5.7 billion in 2021 and is projected to exceed $12.2 billion by 2030 according to Vision Research.
And next to the increasingly addressable market as the global population ages, there's another critical factor that makes Parkinson's disease an attractive area for investment.
There hasn't been a breakthrough in treatment of Parkinson’s since levodopa was put into use and no treatment can alter the course of disease for these patients!
Considered a wonder drug in the 1960s, Levodopa became the first-line treatment for the disease due to its action of entering the brain and helping replace the lost dopamine.
Inhibikase Therapeutics, Inc. (Nasdaq: IKT) is working to change the slow progress in treating Parkinson’s disease by developing disruptive therapies!

Who is IKT?
Inhibikase Therapeutics, Inc. (Nasdaq: IKT) is developing treatments that seek to intervene in neurodegeneration where disease begins.
The company’s treatments flow from a novel understanding of the etiology—cause or set of causes—of neurodegenerative diseases and neurological infections.
IKT targets a key event, the point at which degeneration is initiated inside the affected neurons, which the company believes initiates the disease.

Reversing the Effects of Neurodegenerative Diseases
The company’s drug development programs focus on halting and reversing the effects of neurodegenerative diseases inside and outside the brain.
IKT’s pipeline includes multiple products developed from the company’s proprietary RAMP™ drug innovation and prodrug technology engines that address multiple indications.
Abl Kinases and CNS Diseases
The protein kinase family known as the Abelson Tyrosine Kinase, or Abl kinases, has been shown to play a critical role in monitoring insults to brain neurons and regulating biological pathways that are associated with neurodegeneration. In addition, recent research has demonstrated that Abl kinases are essential checkpoint regulators that play a central role in Parkinson’s disease initiation and progression.
The breakthrough cancer treatment, imatinib (marketed as Gleevec®), was the first FDA-approved Abl kinase inhibitor. However, imatinib cannot treat in the brain nor inhibit the wildtype enzyme in patients, even though it has a favorable safety and pharmacology profile.
Applying IKT’s RAMP™ technology to imatinib, Inhibikase generated novel chemical entities that have:
- Up to 25-fold enhanced potency against wildtype c-Abl relative to imatinib
- Cross the blood-brain barrier and treat in the brain
- Nearly identical route of metabolism to imatinib, thereby preserving the relationship between administered dose, drug safety and frequency of adverse events. This provides a level of predictability in a novel drug’s safety profile.
The company believes these molecules have the potential to be dosed at low levels with an improved safety profile relative to other drugs in this class currently used to treat certain forms of blood and gastrointestinal cancers, resulting in a potentially safer Abl kinase inhibitor that could be chronically and systemically administered for the treatment of our CNS indications.

Abl Kinases and Autonomic Nervous System Diseases
Parkinson’s patients experience a series of disorders that could be the earliest manifestations of the disease. These include difficulty in swallowing (dysphagia) and difficulty in passing solid waste (neurogenic constipation), difficulty in controlling heart rate and blood pressure, along with many other autonomic functions. These arise due to a loss of function in neurons inside or outside the brain necessary for these functions to occur.

IKT’s pre-clinical animal model studies suggest that it is possible to halt the progression of neurodegeneration inside and outside of the brain to restore normal neurological function!
The RAMP™ Drug Innovation Engine
IKT’s proprietary Re-engineering Approach with Metabolism Preserved (RAMP™) drug innovation engine enables us to design and develop new chemical entities that draw on clinical experience to preserve the desired properties of template kinase inhibitor while ameliorating undesired properties, such as toxicity.
Basically, the company is building a better mousetrap.
The company selects clinically validated kinase inhibitors as templates. From these templates, it seeks to create novel drugs with enhanced penetration into the brain, with potentially higher potency against the target and improved safety, to treat neurological and non-neurological diseases in which kinase activation is implicated. They are designed to be administered chronically and systemically.
Small-molecule Medications with World-class Potential
Inhibikase Therapeutics’ first product candidates were generated using the company’s Re-engineering Approach with Metabolism Preserved (RAMP™) drug innovation engine use the same targeting strategy—blocking the activation of Abl kinases—as the basis for small-molecule medications capable of halting the progression of diseases across the therapeutic landscape.
Risvodetinib:
A New Approach to Altering the Course of Parkinson’s Disease
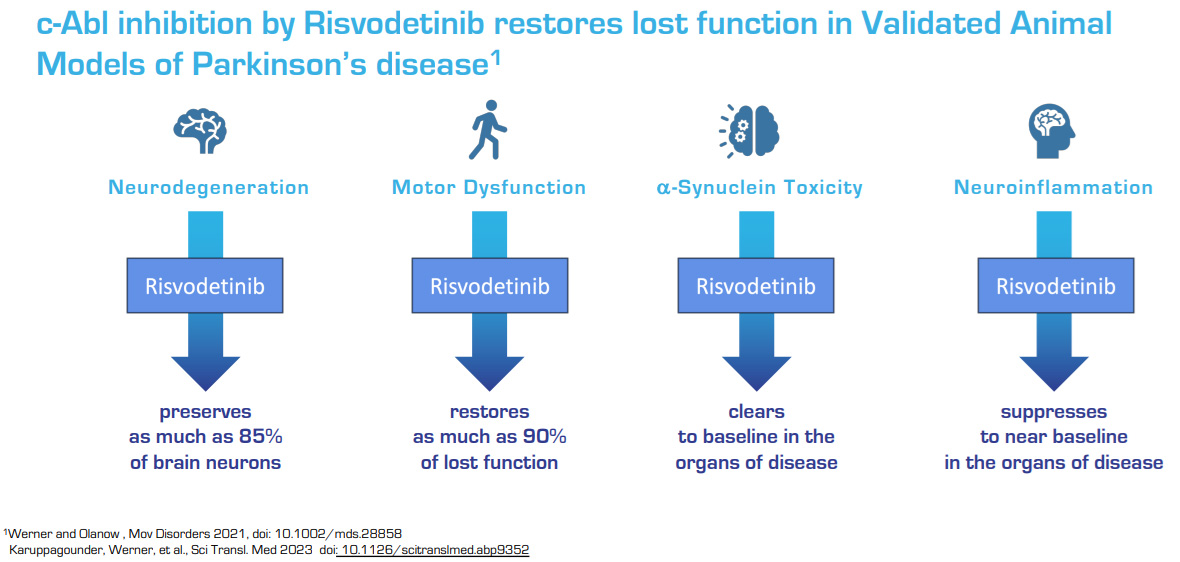
Risvodetinib (IkT-148009), is a potent, selective small-molecule medication designed and engineered as chronically administered, once-daily oral medication targeting the underlying biological mechanism resulting in Parkinson’s disease, with the goal of halting disease progression and reversing functional loss.
Risvodetinib (IkT-148009) is designed to block the activation of Abl kinase, a clinically validated drug target, to halt and reverse the loss of dopamine-secreting neurons in the brain and GI tract by restoring neuroprotective mechanisms.
Parkinson’s patients have a series of disorders that may represent the earliest manifestations of the disease. These include difficulty in swallowing (dysphagia) and difficulty passing solid waste (neurogenic constipation), both due to a loss of the neurons necessary for these functions to occur on their own.
IKT believes that its recent analysis of GI functional disorders in preclinical animal models with its lead product candidate for Parkinson’s, Risvodetinib (IkT-148009), suggests that the company can halt the progression of neurodegeneration in the GI tract and restore the functional loss.
Having demonstrated that Abl kinase inhibition can protect against disruption of development and progression of Parkinson’s-like disease in the brain and GI tract of validated animal models, the company has submitted two Investigational New Drug (IND) applications in February, 2019 to begin clinical development in multiple classes of Parkinson’s patients. Clinical development should proceed through 2024.
The 201 Clinical Trial For Patients With Untreated Parkinson’s Disease
The 201 Clinical Trial is a trial that will explore whether a novel drug, Risvodetinib (IkT-148009), can slow or halt progression of Parkinson’s disease. The trial will also evaluate whether Risvodetinib (IkT-148009) can affect disease progression over three months and fifteen months in two phases.
Brief Summary
This study investigates the safety and tolerability of drug IkT-148009 in untreated Parkinson's disease volunteers (30 to 80 years old). It also looks at the pharmacokinetics of IkT-148009 in the body and evaluates the effect of risvodetinib on motor and non-motor features of the disease. This 12 week study is designed to be 3:1 randomized across 3 doses of risvodetinib or placebo. Each participant will self-administer one of 3 doses or placebo of risvodetinib once daily (QD) with food for 12 weeks. For more information, visit website: www.the201trial.com
Detailed Description
This is a 12-Week, randomized, double-blind, multi-center, placebo-controlled dose-ranging clinical trial of three risvodetinib doses in patients with untreated PD designed to assess safety, tolerability, and pharmacokinetics of risvodetinib, an oral, once daily c-Abl tyrosine kinase inhibitor. Secondary and exploratory assessments will evaluate the effect of IkT-148009 on motor and non-motor features of the disease. 120 participants are anticipated to be enrolled at up to 34 sites across the US.
As of January 30th, 2024, 32 sites are open and actively evaluating prospective trial participants. 49 participants have been enrolled, 20 prospective participants are in medical screening and 52 potential participants are being evaluated for suitability to initiate medical screening.
The 201 Trial patient portal (www.the201trial.com) has been visited by more than 20,000 unique people since launch in September, 2023. The pre-qualification process has led to more than 250 unique individuals to contact open clinical sites, the first step in the evaluation process that could lead to enrollment. Monthly site enrollments have increased month-over-month since this patient outreach program was initiated.
Unblinded Functional Analysis from the 201 Trial Are Encouraging
In August 2023, unblinded functional assessments of 11 patients with untreated Parkinson’s disease were presented at the Movement Disorder Congress in Copenhagen, Denmark. These participants were withdrawn from the trial following the FDA’s temporary clinical hold in November, 2022, which was lifted January, 2023.
These assessments showed that patients receiving the 200 mg dose of risvodetinib (N=3) saw a greater than 10 point improvement over placebo in the sum of motor and non-motor scores after once daily treatment for up to 11 weeks; by contrast, a typical patient with Parkinson’s disease would worsen by 3 to 6 points in the sum of motor and non-motor score assessments over a 12 month period.
While the number of treated participants is too small for the Company to conclude a clinical benefit, these early data are cautiously encouraging.
Orphan Drug Designation in Multiple System Atrophy (MSA)
In October 2023, risvodetinib was granted Orphan Drug Designation by the FDA for the treatment of MSA. In animal model studies of MSA, risvodetinib was shown to be therapeutically active.
The designation by the FDA underscores the need for innovative treatment options for patients afflicted with this rare and rapidly progressing Parkinson’s-related disorder.
The Company is pursuing a comparable designation in the European Union, or E.U., as part of its efforts to initiate a Phase 2 clinical trial to evaluate risvodetinib in MSA. The Company is discussing conduct of the trial with private foundations, Federal and industry stakeholders in an effort to initiate this trial in the future.
IkT-001Pro for Chronic Myeloid Leukemia (CML): A New and Improved, Safer Alternative to Imatinib
IKT has developed IkT-001Pro as an outgrowth of its efforts to improve the safety of the first FDA-approved Abl kinase inhibitor, imatinib (marketed as Gleevec®)!
Imatinib is commonly taken for hematological cancers that arise from Abl kinase mutations in the bone marrow or for gastrointestinal cancers that arise from c-Kit mutations in the stomach.

- IkT-001Pro has shown to be potentially as much as three times safer than imatinib in non-human primates, blunting gastrointestinal side effects of imatinib therapy that occur following oral administration. Removing these gastrointestinal side effects has the potential to significantly improve the number of patients that reach and sustain complete cytogenetic response in stable-phase chronic myelogenous leukemia (CML). IKT believes that removing these side effects will also improve patient adherence to daily therapy and improve patients' quality of life while on therapy.
- IkT-001Pro has received Orphan Drug Designation for stable-phase CML and will follow the development pathway for approval through the 505(b)(2) regulation. This pathway would allow the company to rely, in part, on data in the public domain or the FDA’s prior conclusions regarding the safety and effectiveness of an approved compound.
- The company’s completed ‘501’ study has identified the doses of IkT-001Pro that reaches the same exposure as the approved doses of 400 mg and 600 mg imatinib mesylate and has demonstrated that there were minimal adverse events observed at all doses. The company believes that this data supports the potential approval of IkT-001Pro with blood or stomach cancers, similar to the adult indications for imatinib mesylate.
- The company was granted a pre-New Drug Application (pre-NDA) meeting that was held with the FDA review team in January 2024 to discuss the requirements for approval of IkT-001Pro and to review the data establishing doses of IkT-001Pro the Company believes are bioequivalent to 400 mg and 600 mg imatinib mesylate.
To Reiterate Why IKT Should Be on Your Radar:
- Multi-therapeutic pipeline across neurodegenerative disease, cancer, and infectious disease.
- Risvodetinib (IkT-148009): Lead Selective Abelson Tyrosine Kinase (c-Abl) inhibitor with potential to be a disease-modifying treatment for Parkinson’s disease (PD) and related disorders. Phase 2, 201 trial ongoing with nearly all sites open, approximately 41% enrolled in the U.S. The 201 trial planned to be expanded for 12 additional months providing 15 months of data to evaluate clinical benefit.
- IkT-001Pro: Prodrug of imatinib mesylate with improved safety/tolerability profile for the treatment of stable-phase chronic myelogenous leukemia (CML). Bioequivalent 501 trial completed and FDA pre-NDA meeting January 2024 to discuss approval for 8 adult indications under 505(b)(2).
- Robust patent portfolio with protection to 2033 (oncology) and 2036 (neurodegeneration). Orphan designations: Rivsodetinib in Multiple System Atrophy, IkT-001Pro in up to 8 oncology indications.
- Cash/cash equivalent runway into 4Q24. Continue to seek non-dilutive capital through Federal Government and private foundation sources.
- Highly experienced management team, consultants, Board of Directors, and Scientific Advisory Board.
The Bottom Line….
Inhibikase Therapeutics, Inc. (Nasdaq: IKT) is a clinical-stage pharmaceutical company developing therapeutics for Parkinson's disease and related disorders.
The company’s multi-therapeutic pipeline focuses on neurodegeneration and its lead program for Risvodetinib, an Abelson Tyrosine Kinase (c-Abl) inhibitor, targets the treatment of Parkinson's disease inside and outside the brain as well as other diseases that arise from Abelson Tyrosine Kinases.
Its multi-therapeutic pipeline is pursuing Parkinson's-related disorders of the brain and GI tract, orphan indications related to Parkinson's disease such as Multiple System Atrophy, and drug delivery technologies for kinase inhibitors such as IkT-001Pro, a prodrug of the anticancer agent imatinib mesylate that the Company believes will provide a better patient experience with fewer on-dosing side-effects.
The Company's RAMP™ medicinal chemistry program has also identified a number of follow-on compounds to IkT-148009 to be potentially applied to other cognitive and motor function diseases of the brain.
There's currently no cure for Parkinson's disease, but treatments are able to relieve the symptoms helping patients maintain a quality of life. IKT’s novel treatments could give hope to many Parkinson’s Disease patients around the world!
Large companies such as pharma giants Merck & Co (NYSE: MRK), Roche (ROG: SIX), Novartis (NOVN: VX), and AbbVie (NYSE: ABBV) are showing greater interest in Parkinson’s Disease treatments.
IKT is anticipating results from its 201 trial may be available in the second half of 2024 and is continuing its efforts to complete the requirements for FDA approval of IkT-001Pro!

Learn More about Inhibikase Therapeutics, Inc. by gaining access to the latest corporate presentation
Download PRESENTATIONTHIS IS A PAID ADVERTISEMENT
NO INVESTMENT ADVICE
disclaimer
