As the biotech space heats up, this undiscovered NASDAQ company could shine as it aims to redefine mental healthcare!

Pasithea Therapeutics Corp. (NASDAQ: KTTA) has a current market cap of only $26M and cash in the bank sits at $52M!
Download Research Report

This May Be One of the Biggest Ground-Floor Narratives Unfolding in the Biotech Arena and You’re Reading About It at the Early Stages!
INTRODUCING:
Timing is everything and now may be one of the most pivotal times to be paying attention to Pasithea Therapeutics Corp. (NASDAQ: KTTA)
BIG NEWS HAS BEEN ANNOUNCED
The company recently announced in a press release that its wholly owned subsidiary, Pasithea Clinics, will open a clinic in Los Angeles, California, to provide intravenous (“IV”) ketamine and other therapies to patients suffering from treatment-resistant mental health issues!
“The opening of our first in-person clinic in the United States is a milestone for the Company. Certain novel approaches in psychiatry cannot be delivered at home, such as repetitive transcranial magnetic stimulation (“rTMS”). This physical clinic will allow us to expand our services beyond IV ketamine therapy and continue to address the important segment of patients who are suffering from treatment resistant mental health disorders. This is a step forward in creating a hybrid model for Pasithea, complementing our at-home services.”
— Dr. Tiago Reis Marques, CEO of Pasithea Therapeutics
Another major highlight of this recent press release is that the company has raised $60 million through offerings with a current cash position of ≥ $52 million!
This is NOT a typo!
This biotech has a market cap of $26M today and along with the strong cash position, is creating a rare opportunity for investors to invest at a 50% discount to the cash value!
Already at the forefront of neuroscience research, U.S.-based Pasithea Therapeutics Corp. (NASDAQ: KTTA) is aiming to develop game changing medicines to solve psychiatric and neurological disorders and could be on the verge of gaining major attention on Wall Street!

Already at the forefront of neuroscience research, U.S.-based Pasithea Therapeutics Corp. (NASDAQ: KTTA) is aiming to develop game changing medicines to solve psychiatric and neurological disorders.
The company is focused on the research and discovery of new treatments in psychiatry medicine while also offering ketamine therapy in their clinics.
KTTA is targeting treatment-resistant depression and PTSD using ketamine infusions. In fact, the company intends to operate mental health clinics using board certified anesthesiologists to administer intravenous infusions of ketamine.
What makes KTTA truly sound out is that they want to deliver ketamine infusions in the comfort of a patient’s home. By eliminating the need to leave the house will be a big relief for millions of people suffering from mental health disorders such as depression!
SEE THE FULL STOCK CHART HERE...
The world is in desperate need for a revolution in mental health care. The status quo today is simply unacceptable. Moving away from traditional practices and thinking may be the only way to bring a sea of change to one of the most important areas in the massive healthcare space.

Psychedelics like ketamine and psilocybin are getting a second look as a way to treat psychiatric problems like depression, anxiety, substance disorders, and PTSD.
The World Health Organization (WHO) has even placed ketamine on its list of essential substances that should be available to patients in any health system!
This is just one reason why KTTA should be high on your radar!!
The company’s research will be conducted under the leadership of Professor Lawrence Steinman, a renowned neurologist and immunologist based at Stanford University and Dr. Tiago Reis Marques, who is a psychiatrist and neuroscientist at Imperial College and King’s College London.
With not one, but TWO, reputable scientists leading the way, KTTA may quickly capture Wall Street attention!
“Our successful IPO is testament to our work as a biotech company paving the way for new solutions to address how we treat mental health disorders.”
— Dr. Tiago Reis Marques, CEO and director of Pasithea
More Reasons to Have KTTA on Your Radar Include:
- Being fresh to the NASDAQ makes this new IPO alert one to pay very close attention to as the world begins to learn about what the company is doing! This small cap company only debuted in September of 2021 on a big board exchange!
- It was also in September that the very first active psychedelics ETF in the country had hit the NYSE! Do you know what this means? It means that not only the world, but Wall Street too, is waking up to the promising benefits of psychedelics in medicine, including ketamine.
- Ketamine has been referred to as the FUTURE of depression treatment! A study at the Karolinska Institute in Sweden was published in the summer of 2020 and has shown that ketamine could work quickly to help those with treatment-resistant depression.
- It was in 2019 that the Food and Drug Administration (FDA) approved the first truly new medication for major depression in decades. The drug is a nasal spray called esketamine, derived from ketamine - an anesthetic that has made waves for its surprising antidepressant effect. Even the FDA is recognizing the benefits!
- Precedence Research projects that the global behavioral health market size is to surpass around US$ 242 billion by the end of 2027 from estimated USD 140.01 billion in 2019!
- As per FutureWise Research, the global resistant depression treatment market is expected to be valued over $1 billion by the end of 2026 with an expected CAGR of 3% from 2019 to 2026!
- The company recently added Esketamine nasal spray to its clinic offerings in the U.K. This is an important milestone for their U.K. clinics and their patients!
The unexplored potential of ketamine could quickly catapult this newly traded NASDAQ company to the forefront….
It was only last September that the company launched a multi-million dollar IPO on the Nasdaq under the symbol “KTTA.”
This is all still very fresh, and this emerging biotech player could be one of the most exciting GROUND-FLOOR stories set to take off!
Keep on reading to learn why Pasithea Therapeutics Corp. (NASDAQ: KTTA) may be one of the most exciting small cap companies to have on your watch list!
Greetings Investors,
Every year, 13 million to 14 million Americans have major depression Of those who seek treatment, 30% to 40% will not get better or fully recover with standard antidepressants.
Sadly, this puts them at greater risk of alcohol and drug abuse, hospitalization, and suicide attempts.
Depression has been alarmingly on the rise in the past year with what the world has gone through. The pandemic has tripled the rate of depression in US adults in all demographic groups— especially in those with financial worries—and the rise is much higher than after previous major traumatic events, according to a study published in JAMA Network Open.
Now, though, a growing body of research shows there may be new hope: the anesthetic drug ketamine.
As stated in the Psyciatric Times, “Ketamine has caused quite a stir in psychiatric practice. Sub-anesthetic administrations of ketamine have been shown to markedly improve symptoms of depression and anxiety!”

Ketamine was only introduced into clinical practice in the 1960’s but STILL continues to be both clinically useful and scientifically fascinating today.
Recreational ketamine use however, is not legal, and is associated with dangers and a serious risk of developing dependence and addiction.
Even though it may get a bad reputation for that reason, ketamine is being recognized as effective in treating some mental illnesses and even substance abuse addictions under medical supervision. Furthermore, the doses used for medical purpose are much lower than the doses used recreationally, and studies have shown that the risk of developing addiction at this dose is absent.
With a lot of research done already, and more in progress, the world is quickly learning that ketamine can be beneficial in healthcare.
It was a huge milestone that a psychedelics ETF hit the NYSE this year!
The AdvisorShares Psychedelics ETF provides exposure to biotechnology, pharmaceutical and life sciences companies that the firm deems are “leading the way in this nascent industry.”
All of this may soon shine a spotlight on Pasithea Therapeutics Corp. (NASDAQ: KTTA) who is quietly emerging as a major player in the biotech industry!

ABOUT THE COMPANY
Pasithea Therapeutics Corp. is a biotechnology company focused on the research and discovery of new and effective treatments for psychiatric and neurological disorders.
As media awareness for new treatments for mental health disorders such as ketamine continues to grow, and more studies show the value that it may have in treating mental disorders, this small-cap company could become a tremendous biotech growth story!
COMPANY HIGHLIGHTS
- Operations are focused on developing drugs that target the pathophysiology underlying psychiatric and neurological disorders and it is developing novel pharmacological agents that have the potential for an increased level of effectiveness on patients suffering from illnesses such as depression, PTSD (post-traumatic stress disorder), schizophrenia, and others.
- The company is currently building its drug development pipeline and will be going through the standard IND (Investigational New Drug) application process. It recently announced that they will progress their first compound in collaboration with Evotec, one of the largest and most well-respected drug discovery and development companies in the world!
- A simultaneous revenue stream is expected to launch soon, in the form of mental health clinics offering intravenous ketamine infusions. The company has already established partnerships in locations across Los Angeles, New York City and London!
The company’s biotech operations will focus on developing drugs that target the pathophysiology underlying such disorders rather than symptomatic treatments, with the goal of developing new pharmacological agents that potentially display significant advantages over conventional therapies with respect to efficacy and tolerability.
The Company’s secondary operations focus on establishing mental health clinics using ketamine infusion therapy in the United Kingdom and in the United States.
The Company’s operations in the United Kingdom will involve providing business support services to registered healthcare providers who will assess patients, and if appropriate, administer intravenous infusions of ketamine, and the Company’s operations in the United States will involve providing business support services to entities that furnish similar services to patients who personally pay for those services.
Who Is Affected by Depression?
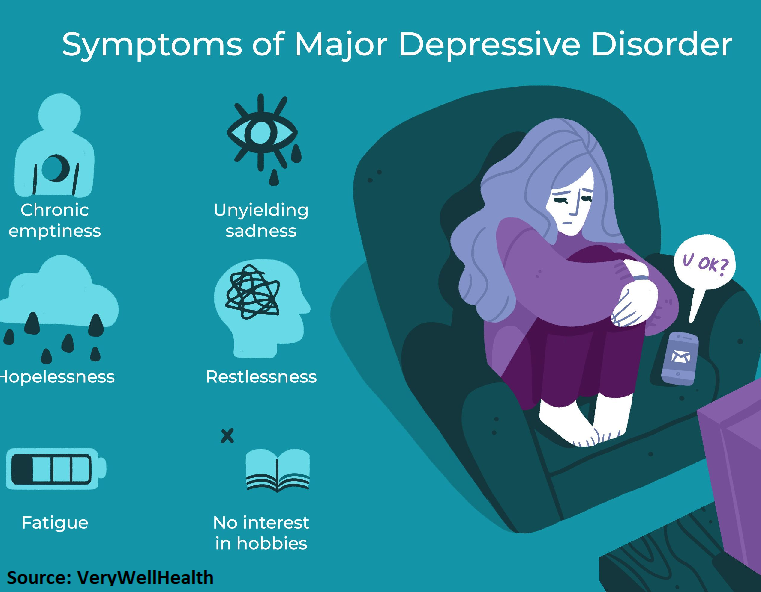
* Major depressive disorder affects approximately 17.3 million American adults, or about 7.1% of the U.S. population age 18 and older, in a given year. (National Institute of Mental Health “Major Depression”, 2017)
* Major depressive disorder is more prevalent in women than in men. (Journal of the American Medical Association, 2003; Jun 18; 289(23): 3095-105)
* 1.9 million children, 3 – 17, have diagnosed depression. (Centers for Disease Control “Data and Statistics on Children’s Mental Health”, 2018)
* Adults with a depressive disorder or symptoms have a 64 percent greater risk of developing coronary artery disease. (National Institute of Health, Heart disease and depression: A two-way relationship, 2017)
Source: https://www.dbsalliance.org/education/depression/statistics/
Research and Markets has projected that the global antidepressants market is expected to grow from $14.3 billion in 2019 to about $28.6 billion in 2020 as mental health issues are expected to surge due to the pandemic.
What is Ketamine?
Ketamine is classified pharmacologically as an uncompetitive NMDA receptor antagonist. This means that Ketamine blocks a brain receptor called NMDA.
Ketamine was synthesized in the 1960´s and approved by the US Food and Drug Administration (FDA) in 1970 to be used as an anesthetic.
More recently, Ketamine has been repurposed for the use in psychiatry and has proven to be effective in the treatment of several psychiatric disorders, such as:
- Treatment Resistant Depression (TRD)
- Post-Traumatic Stress Disorder (PTSD)
For these disorders, Ketamine is used at lower doses, below the anesthetic threshold.
How Does Ketamine Work?
The dominant view on how Ketamine produces its antidepressant effects (often referred to as “mechanism of action”), is that Ketamine promotes and facilitates synaptogenesis (the formation of new synapses).
It does this by blocking the NMDA receptors which are located on inhibitory neurons, called GABA interneurons. When Ketamine blocks the NMDA receptor, the inhibitory effect of the GABA interneurons disappears. In turn, this leads to an increase in the firing of another set of neurons called pyramidal cells with a consequent final increase in glutamate levels. Glutamate is a neurotransmitter and the primary excitatory neurotransmitter in the brain.
Therefore, the NMDA receptor plays a pivotal role in the synaptic transmission and synaptic plasticity. Contrary to conventional antidepressants, Ketamine does not act within weeks, but may act much faster, occasionally even in 24 hours. Therefore, it has been suggested to be a different class of antidepressant, called a fast-acting antidepressant.
There is also evidence that Ketamine's mechanism of action can extend beyond glutamate and impact on other neurotransmitter systems. However, more research is needed to fully understand which mechanisms contribute to the antidepressant and psychiatric benefits of Ketamine.
Which Ketamine?
Chemically, Ketamine consists of a mix of two versions of the same compound, ‘r-ketamine’ and ‘s-ketamine’ (sometimes referred to as ‘esketamine’). In KTTA's clinic, the company uses the ‘standard’ ketamine, i.e. the mixture of r- and s- ketamine (also called racemic ketamine).
A recent meta-analysis, (a statistical analysis which combines the results of multiple scientific studies), has assessed the comparative efficacy of esketamine and ketamine for the treatment of depression. 24 studies, representing 1877 patients, were included in this study. Results showed that racemic ketamine, relative to esketamine, demonstrated a greater overall treatment response as well as improved remission rates.
Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: A systematic review and meta-analysis. Journal of Affective Disorders. 2021 Jan 1;278:542-555.
Who is Eligible to Receive Ketamine?
Ketamine is a new treatment for people who have been suffering from mental health disorders such as depression and are still unwell despite having received adequate medication (i.e. people who have ‘Treatment-Resistant Depression’ or TRD for short).
Before a person starts the Ketamine treatment, they will have a full assessment by a psychiatrist to ensure that Ketamine is likely to help with their illness and is going to be safe for them.
If the person suffers from other psychiatric problems such as PTSD or Anxiety Disorder, they will be able to discuss this with the company’s psychiatric team and they will confirm their eligibility. If their psychiatrist considers them to be a suitable candidate for Ketamine treatment, the company’s team will contact them and discuss the treatment.
There are also some contra-indications to the use of ketamine, such as having a history of psychosis, traumatic brain injury, active substance use or allergy to Ketamine. The company team will review a person’s medical history and they will have the opportunity to discuss their eligibility for treatment.

UNITED STATES
In the United States, Pasithea Therapeutics Corp. has entered an exclusive agreement with IV Doc, one of the largest mobile medical infusions companies, to be able to deliver ketamine infusions in the comfort of a patient’s home.
All ketamine treatments are provided by board-certified medical staff. This will allow Pasithea Therapeutics Corp. to operate in a safe and capital-light way and quickly expand throughout the major cities in the US!
Many psychiatric patients experience symptoms such as social isolation, acute anxiety, lethargy, and lack of motivation. By offering ketamine infusion treatment at the patient's house, the company believes it is providing a service to many patients who struggle to leave their own accommodations, and without the constraints associated with visiting a clinic.
The United Kingdom
In the United Kingdom, Pasithea Therapeutics Corp. has an exclusive agreement with Zen Healthcare, a large healthcare group with clinics in the most affluent areas of London. Pasithea will be one of the few clinics in all of the UK providing this innovative treatment.
The company’s wholly owned subsidiary, Pasithea Clinics, has been approved to provide esketamine nasal spray (SPRAVATO®) for treatment-resistant depression in adults, and has begun offering the treatment in its Knightsbridge, London location. Only three clinics in the U.K. have been accredited to offer this treatment.
“This is an important milestone for our U.K. clinics and their patients. Major Depression is the leading cause of long-term disability worldwide. Current treatments have limited success and up to 30% of patients with depression do not respond to consecutive trials of antidepressant treatment. These patients are considered to have treatment-resistant depression and new treatment options are urgently needed.”
— Dr. Tiago Reis Marques, CEO of Pasithea Therapeutics
“Esketamine is safe and effective, especially when combined with ongoing psychiatric support. Due to some risks associated with this drug, patients treated in outpatient settings must be enrolled in a specific program. We are extremely proud to have been accredited to provide this treatment, a reflection of our high standards of care.”
— Dr. Yassine Bendiabdallah, Managing Director of Pasithea Clinics in the U.K.
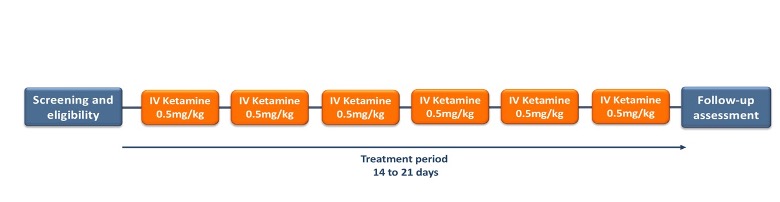
HOW THE PROGRAM WORKS
For each patient, the program will consist of three phases:
- Screening phase
- Treatment phase
- Follow-up assessment
Screening phase
During this phase patients will be screened by a psychiatrist through a video consultation. A company psychiatrist will see if you are eligible to receive IV ketamine, according to their inclusion and exclusion criteria (link to section: about-iv-ketamine /who is eligible to receive ketamine).
Treatment phase
The treatment consists of the following: consent review with the treating provider, EKG, Weight, Vital Signs, urine drug screen and a pregnancy test for female patients. If remaining within the inclusion criteria, a cannula will be placed and a slow infusion of ketamine over 30-40 min will ensue. A clinician will be at the bedside throughout the infusion and the patient will remain under observation until the acute effects have worn off.
During a session, the patient will experience a degree of sedation, or sleepiness, and a degree of dissociation, i.e. any of a number of experiences, such as feeling detached from reality, feeling in a dream-like state or seeing things move more slowly than usual. Dissociative experiences may feel positive or negative. A clinician will be with the patient while they experience these effects and will remain with them until they have worn off and discharged from the day of service. The patient may be accompanied by someone during the sessions. To minimize the potential impact of sedation and dissociation, you should abstain from drinking alcohol during the course of your treatment. In addition, the patient will be advised not to drive, operate machinery or do anything else that requires your full alertness, until the day after a ketamine session and after a period of restful sleep.
Follow-up assessment
A final Psychiatric consultation via telemedicine / video consult following your last IV Ketamine treatment. During this final session you will be assess on how IV ketamine benefited you and our treating psychiatrist will determine if more IV ketamine infusions are required, maintenance dosing, further counseling, or other referral.
KETAMINE in the News
* Revisiting the Hallucinogenic Potential of Ketamine (psychiatrictimes.com)
- We Now Know Why Ketamine Is So Effective At Treating Depression | IFLScience
- Ketamine Relieves Depression By Restoring Brain Connections : Shots - Health News : NPR
- Yale: 'Magic' Antidepressant May Hold Promise For PTSD - Hartford Courant
- Ketamine reduces muscle pain, temporal summation, and referred pain in fibromyalgia patients - ScienceDirect
KETAMINE Research
- Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression - PubMed (nih.gov)
- Ketamine and the next generation of antidepressants with a rapid onset of action - ScienceDirect
- Antidepressant effects of ketamine in depressed patients - PubMed (nih.gov)
- Use of Ketamine in Acute Cases of Suicidality (nih.gov)
- Efficacy of Intravenous Ketamine for Treatment of Chronic Posttraumatic Stress Disorder: A Randomized Clinical Trial | Anesthesiology | JAMA Psychiatry | JAMA Network
THE COMPANY TEAM
Leadership Earns Industry-Wide Respect from Publishing and Research Background in Field!

Dr. Tiago Reis Margues
CEO of Pasithea Therapeutics and Leading Psychiatrist
Dr. Marques is a fellow at Imperial College London and a lecturer at the Institute of Psychiatry, King’s College London. Dr. Marques is also a psychiatrist at Maudsley Hospital, rated as one of the top three psychiatry centres in the world. His research focuses on topics including the mechanism of action of psychiatric medication and the unveil of novel treatment targets. Dr. Marques has co-authored international treatment guidelines and written book chapters, including in the leading book in the field, “Neurobiology of Mental Illness.”

Prof. Lawrence Steinman
Chairman
Company Chairman is Prof. Lawrence Steinman is a professor of Neurology and Neurological Sciences, Pediatrics, and Genetics at Stanford University Medical School. He is also a member of the National Academy of Sciences and the National Academy of Medicine. His research in multiple sclerosis culminated in a potent research on MS, and the Charcot Prize for Lifetime Achievement in MS research.

Dr. Elliot Nadelson
Managing Director of Pasithea Clinics USA
Dr. Nadelson is an American Board-Certified Urologist and the co-founder and CEO of The I.V. Doc®. The IV Doc software and technology platform has enabled their clinical affiliates to treat over 50,000 patients and establish relationships with over 800 clinicians over the past seven years.
Additional accolades from management include:
CEO Dr. Tiago Reis Marques is an academic psychiatrist at the Faculty of Medicine at Imperial College and the Institute of Psychiatry, Psychology & Neuroscience (IOPPN) at King’s College London. The IOPPN produces more highly cited outputs (top 1% citations) on mental health than any other centre in the word! He has also authored of over 100 scientific publications in peer-reviewed psychiatry and neuroscience journals.
THE BOTTOM LINE
Miami-based biotech company Pasithea Therapeutics Corp. (NASDAQ: KTTA), which closed its approximately $24 million Initial Public Offering (IPO) in September — has now joined the ranks of other recent medical IPOs such as Monte Rosa Therapeutics Inc. (NASDAQ: GLUE) and Ambrx Biopharma Inc - ADR (NYSE: AMAM).
The company is zooming in on the massive mental health care market, in particular depression - a leading cause of disability. Depression is a major contributor to the disease burden worldwide and the global prevalence of depression and depressive symptoms has been increasing in recent decades.
Ketamine is an already FDA-approved drug and has been used for many years as an anesthetic. However, it has recently been repurposed for the treatment of psychiatric disorders using significantly lower doses than other forms of anesthesia, with remarkable efficacy.
Pasithea Therapeutics Corp. (NASDAQ: KTTA) is offering ketamine infusions in the comfort of peoples’ homes, giving it a huge advantage in a niche industry that is expected to become a big part of healthcare!
Depression is the leading cause of disability and is a major contributor to The biotech sector is one of the hottest sectors on Main Street and finding a ground-floor story like KTTA at the first chapter should not be dismissed!
The biotech industry saw a big boom which began early last year as the pandemic set in. And while it started with an emphasis on biotech companies working on a treatment or vaccine, it quickly spread to other companies too.
This momentum is still sky high in the arena and KTTA should be high on your radar for the promising growth potential that lies here!
Hurry and start your research!
This website is wholly owned by scd media llc (d/b/a “smallcapsdaily.com”). Our reports are advertorials and are for general information purposes only. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. This disclaimer is to be read and fully understood before using our services, joining our email list, as well as any social networking platforms we may use. Please note as well: Small Caps Daily and its employees are not Registered Investment Advisors, broker-dealers, or member(s) of any association for other research providers in any jurisdiction whatsoever. release of liability: through use of this website, viewing or using you agree to hold Small Caps Daily, its operators, owners, and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources that we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. Small Caps Daily encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the company profiled or is available from public sources and Small Caps Daily makes no representations, warranties, or guarantees as to the accuracy or completeness of the disclosure by the profiled company. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provided herein. Instead, Small Caps Daily strongly urges you to conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Small Caps Daily’s full disclosure is to be read and fully understood before using Small Caps Daily's website, or joining Small Caps Daily's email or text list. From time to time, Small Caps Daily will disseminate information about a company via website, email, sms, and other points of media. By viewing Small Caps Daily's website and/or reading Small Caps Daily's email or text newsletter you are agreeing to this ----> https://smallcapsdaily.com/disclaimer/. All potential percentage gains discussed in any communications are based on calculations from the low to the high of the day. We are engaged in the business of marketing and advertising companies for monetary compensation. In compliance with section 17(b) of the securities act we are disclosing that we have been compensated a fee pursuant to an agreement between scd media llc and sea path advisory. Small Caps Daily was hired for a period beginning january 2022 and ending march 2022 to publicly disseminate information about Pasithea Therapeutics Corp. via website, email, and sms. We were paid five thousand usd via ACH. We are also disclosing that Tradigital Marketing Group has been compensated a fee pursuant to an agreement between Tradigital and Pasithea Therapeutics Corp. Tradigital was hired for a period beginning january 2022 and ending march 2022 to publicly disseminate information about Pasithea Therapeutics Corp., via website, email, and SMS. Tradigital was paid three hundred ninety-four thousand USD via ACH. Subsequently, Tradigital was paid four hundred ninety-nine thousand USD via ACH. Tradigital owns one hundred fifty thousand restricted common shares of Pasithea Therapeutics Corp., which are eligible for sale on 03/18/2022. For the purpose of this disclaimer, we suggest that you assume we will sell all of our shares once the restriction is lifted on 03/18/2022. Readers are advised to review sec periodic reports: forms 10-q, 10k, form 8-k, insider reports, forms 3, 4, 5 schedule 13d. Small Caps Daily is compliant with the can-spam act of 2003. Small Caps Daily does not offer investment advice or analysis, and Small Caps Daily further urges you to consult your own independent tax, business, financial, and investment advisors. investing in micro-cap, small-cap, and growth securities is highly speculative and carries an extremely high degree of risk. it is possible that an investor's investment may be lost or impaired due to the speculative nature of the companies profiled.the private securities litigation reform act of 1995 provides investors a safe harbor in regard to forward-looking statements. any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events, or performance are not statements of historical fact may be forward-looking statements. forward-looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. forward-looking statements in this action may be identified through the use of words such as projects, foresee, expects, will, anticipates, estimates, believes, understands, or that by statements indicating certain actions & quotes; may, could, or might occur. understand there is no guarantee past performance will be indicative of future results in preparing this publication, Small Caps Daily has relied upon information supplied by its clients, as well as its clients’ publicly available information and press releases which it believes to be reliable; however, such reliability can not be guaranteed. investors should not rely on the information contained on this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. the advertisements in this website are believed to be reliable, however, Small Caps Daily and its owners, affiliates, subsidiaries, officers, directors, representatives, and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of material facts from such advertisement. Small Caps Daily is not responsible for any claims made by the companies advertised herein, nor is Small Caps Daily responsible for any other promotional firm, its program, or its structure. Small Caps Daily is not affiliated with any exchange, electronic quotation system, the securities exchange commission, or finra.
THIS WEBSITE IS WHOLLY OWNED BY TRADIGITAL MARKETING GROUP, INC. (D/B/A “TRADIGITAL IR”). OUR REPORTS ARE ADVERTORIALS AND ARE FOR GENERAL INFORMATION PURPOSES ONLY. NEVER INVEST IN ANY STOCK FEATURED ON OUR SITE OR EMAILS UNLESS YOU CAN AFFORD TO LOSE YOUR ENTIRE INVESTMENT. THE DISCLAIMER IS TO BE READ AND FULLY UNDERSTOOD BEFORE USING OUR SERVICES, JOINING OUR EMAIL LIST, AS WELL AS ANY SOCIAL NETWORKING PLATFORMS WE MAY USE. PLEASE NOTE WELL: TRADIGITAL IR AND ITS EMPLOYEES ARE NOT REGISTERED INVESTMENT ADVISORS, BROKER-DEALERS, OR MEMBER(S) OF ANY ASSOCIATION FOR OTHER RESEARCH PROVIDERS IN ANY JURISDICTION WHATSOEVER. RELEASE OF LIABILITY: THROUGH USE OF THIS WEBSITE, VIEWING OR USING YOU AGREE TO HOLD TRADIGITAL IR, ITS OPERATORS, OWNERS, AND EMPLOYEES HARMLESS AND TO COMPLETELY RELEASE THEM FROM ANY AND ALL LIABILITY DUE TO ANY AND ALL LOSS (MONETARY OR OTHERWISE), DAMAGE (MONETARY OR OTHERWISE), OR INJURY (MONETARY OR OTHERWISE) THAT YOU MAY INCUR. THE INFORMATION CONTAINED HEREIN IS BASED ON SOURCES THAT WE BELIEVE TO BE RELIABLE BUT IS NOT GUARANTEED BY US AS BEING ACCURATE AND DOES NOT PURPORT TO BE A COMPLETE STATEMENT OR SUMMARY OF THE AVAILABLE DATA. TRADIGITAL IR ENCOURAGES READERS AND INVESTORS TO SUPPLEMENT THE INFORMATION IN THESE REPORTS WITH INDEPENDENT RESEARCH AND OTHER PROFESSIONAL ADVICE. ALL INFORMATION ON FEATURED COMPANIES IS PROVIDED BY THE COMPANIES PROFILED OR IS AVAILABLE FROM PUBLIC SOURCES AND TRADIGITAL IR MAKES NO REPRESENTATIONS, WARRANTIES, OR GUARANTEES AS TO THE ACCURACY OR COMPLETENESS OF THE DISCLOSURE BY THE PROFILED COMPANIES. NONE OF THE MATERIALS OR ADVERTISEMENTS HEREIN CONSTITUTE OFFERS OR SOLICITATIONS TO PURCHASE OR SELL SECURITIES OF THE COMPANIES PROFILED HEREIN AND ANY DECISION TO INVEST IN ANY SUCH COMPANY OR OTHER FINANCIAL DECISIONS SHOULD NOT BE MADE BASED UPON THE INFORMATION PROVIDED HEREIN. INSTEAD, TRADIGITAL IR STRONGLY URGES YOU TO CONDUCT A COMPLETE AND INDEPENDENT INVESTIGATION OF THE RESPECTIVE COMPANIES AND CONSIDERATION OF ALL PERTINENT RISKS. TRADIGITAL IR’S FULL DISCLOSURE IS TO BE READ AND FULLY UNDERSTOOD BEFORE USING TRADIGITAL IR'S WEBSITE, OR JOINING TRADIGITAL IR'S EMAIL OR TEXT LIST. FROM TIME TO TIME, TRADIGITAL IR WILL DISSEMINATE INFORMATION ABOUT A COMPANY VIA WEBSITE, EMAIL, SMS, AND OTHER POINTS OF MEDIA. BY VIEWING TRADIGITAL IR'S WEBSITE AND/OR READING TRADIGITAL IR'S EMAIL OR TEXT NEWSLETTER YOU ARE AGREEING ----> HTTPS://TRADIGITALIR.COM/DISCLAIMER-TMG/. ALL POTENTIAL PERCENTAGE GAINS DISCUSSED IN ANY COMMUNICATIONS ARE BASED ON CALCULATIONS FROM THE LOW TO THE HIGH OF THE DAY. WE ARE ENGAGED IN THE BUSINESS OF MARKETING AND ADVERTISING COMPANIES FOR MONETARY COMPENSATION. IN COMPLIANCE WITH SECTION 17(B) OF THE SECURITIES ACT WE ARE DISCLOSING THAT WE HAVE BEEN COMPENSATED A FEE PURSUANT TO AN AGREEMENT BETWEEN TRADIGITAL AND PASITHEA THERAPEUTICS CORP. TRADIGITAL WAS HIRED FOR A PERIOD BEGINNING JANUARY 2022 AND ENDING MARCH 2022 TO PUBLICLY DISSEMINATE INFORMATION ABOUT PASITHEA THERAPEUTICS CORP. VIA WEBSITE, EMAIL, AND SMS. WE WERE PAID FIVE HUNDRED FORTY-FOUR THOUSAND USD VIA ACH. WE OWN ONE HUNDRED FIFTY THOUSAND RESTRICTED COMMON SHARES OF PASITHEA THERAPEUTICS CORP., WHICH ARE ELIGIBLE FOR SALE ON 03/18/2022. FOR THE PURPOSE OF THIS DISCLAIMER, WE SUGGEST THAT YOU ASSUME WE WILL SELL ALL OF OUR SHARES ONCE THE RESTRICTION IS LIFTED ON 03/18/2022. READERS ARE ADVISED TO REVIEW SEC PERIODIC REPORTS: FORMS 10-Q, 10K, FORM 8-K, INSIDER REPORTS, FORMS 3, 4, 5 SCHEDULE 13D. TRADIGITAL IR IS COMPLIANT WITH THE CAN-SPAM ACT OF 2003. TRADIGITAL IR DOES NOT OFFER INVESTMENT ADVICE OR ANALYSIS, AND TRADIGITAL IR FURTHER URGES YOU TO CONSULT YOUR OWN INDEPENDENT TAX, BUSINESS, FINANCIAL, AND INVESTMENT ADVISORS. INVESTING IN MICRO-CAP, SMALL-CAP, AND GROWTH SECURITIES IS HIGHLY SPECULATIVE AND CARRIES AN EXTREMELY HIGH DEGREE OF RISK. IT IS POSSIBLE THAT AN INVESTORS INVESTMENT MAY BE LOST OR IMPAIRED DUE TO THE SPECULATIVE NATURE OF THE COMPANIES PROFILED.THE PRIVATE SECURITIES LITIGATION REFORM ACT OF 1995 PROVIDES INVESTORS A SAFE HARBOR IN REGARD TO FORWARD-LOOKING STATEMENTS. ANY STATEMENTS THAT EXPRESS OR INVOLVE DISCUSSIONS WITH RESPECT TO PREDICTIONS, EXPECTATIONS, BELIEFS, PLANS, PROJECTIONS, OBJECTIVES, GOALS, ASSUMPTIONS OR FUTURE EVENTS, OR PERFORMANCE ARE NOT STATEMENTS OF HISTORICAL FACT MAY BE FORWARD-LOOKING STATEMENTS. FORWARD-LOOKING STATEMENTS ARE BASED ON EXPECTATIONS, ESTIMATES, AND PROJECTIONS AT THE TIME THE STATEMENTS ARE MADE THAT INVOLVE A NUMBER OF RISKS AND UNCERTAINTIES WHICH COULD CAUSE ACTUAL RESULTS OR EVENTS TO DIFFER MATERIALLY FROM THOSE PRESENTLY ANTICIPATED. FORWARD-LOOKING STATEMENTS IN THIS ACTION MAY BE IDENTIFIED THROUGH THE USE OF WORDS SUCH AS PROJECTS, FORESEE, EXPECTS, WILL, ANTICIPATES, ESTIMATES, BELIEVES, UNDERSTANDS, OR THAT BY STATEMENTS INDICATING CERTAIN ACTIONS & QUOTES; MAY, COULD, OR MIGHT OCCUR. UNDERSTAND THERE IS NO GUARANTEE PAST PERFORMANCE WILL BE INDICATIVE OF FUTURE RESULTS IN PREPARING THIS PUBLICATION, TRADIGITAL IR HAS RELIED UPON INFORMATION SUPPLIED BY ITS CLIENTS, AS WELL AS ITS CLIENTS’ PUBLICLY AVAILABLE INFORMATION AND PRESS RELEASES WHICH IT BELIEVES TO BE RELIABLE; HOWEVER, SUCH RELIABILITY CAN NOT BE GUARANTEED. INVESTORS SHOULD NOT RELY ON THE INFORMATION CONTAINED ON THIS WEBSITE. RATHER, INVESTORS SHOULD USE THE INFORMATION CONTAINED IN THIS WEBSITE AS A STARTING POINT FOR DOING ADDITIONAL INDEPENDENT RESEARCH ON THE FEATURED COMPANIES. THE ADVERTISEMENTS IN THIS WEBSITE ARE BELIEVED TO BE RELIABLE, HOWEVER, TRADIGITAL IR AND ITS OWNERS, AFFILIATES, SUBSIDIARIES, OFFICERS, DIRECTORS, REPRESENTATIVES, AND AGENTS DISCLAIM ANY LIABILITY AS TO THE COMPLETENESS OR ACCURACY OF THE INFORMATION CONTAINED IN ANY ADVERTISEMENT AND FOR ANY OMISSIONS OF MATERIALS FACTS FROM SUCH ADVERTISEMENT. TRADIGITAL IR IS NOT RESPONSIBLE FOR ANY CLAIMS MADE BY THE COMPANIES ADVERTISED HEREIN, NOR IS TRADIGITAL IR RESPONSIBLE FOR ANY OTHER PROMOTIONAL FIRM, ITS PROGRAM, OR ITS STRUCTURE. TRADIGITAL IR IS NOT AFFILIATED WITH ANY EXCHANGE, ELECTRONIC QUOTATION SYSTEM, THE SECURITIES EXCHANGE COMMISSION, OR FINRA.