This Clinical Stage Biotech Company Focuses on the Development of Regenerative Medicine Treatments to Meet Critical Unmet Patient Needs Across Therapeutic Areas and Create Considerable Market Opportunities …
Introducing: Longeveron Inc. (NASDAQ: LGVN) a clinical-stage biotechnology company developing regenerative medicines for unmet medical needs.
The unmet need for treatment in regenerative diseases presents a pressing issue with a multi-billion dollar market potential.
Regenerative diseases, characterized by the degeneration of tissues and organs, have long been a challenge for the medical community. The lack of effective treatments has created an enormous unmet need, leaving patients with limited options for managing their conditions.

Download Research Report
This unmet need presents an opportunity for innovative companies like Longeveron Inc. (NASDAQ: LGVN).
With its focus on developing regenerative medicines for unmet medical needs, Longeveron is strategically positioned to tap into the growing market demand.
Within this vast market, LGVN has identified specific areas of high unmet need, including Hypoplastic Left Heart Syndrome (HLHS), Alzheimer's disease, and aging-related frailty.
HLHS, a congenital heart defect predominantly affecting infants is one area where Longeveron's regenerative therapies, if successful, could make a substantial impact while capturing a significant patient share.
Alzheimer's disease alone represents a multi-billion dollar market opportunity, with the global market projected to reach USD 18.46 Billion by 2029.
Moreover, the market for geriatric care services, including frailty management, is estimated to reach over 1.6 BN by 2028 fueled by the increasing aging population and the demand for specialized care. Longeveron's focus on addressing aging-related conditions will help position itself to potentially capitalize on this growing market segment.
Longeveron hopes to advance the regenerative medicine market by targeting these unmet medical needs.
LGVN is committed to being a leader in the development of MSCs for therapeutic indications, with a mission to deliver regenerative medical therapies for unmet medical needs.
At the forefront of Longeveron's research is their lead investigational product, Lomecel-B™.
Derived from culture-expanded medicinal signaling cells (MSCs), Lomecel-B™
is currently undergoing evaluation in multiple clinical trials, addressing aging-related chronic diseases and other life-threatening conditions. These trials are conducted under Investigational New Drug (IND) Applications filed by Longeveron with the US Food and Drug Administration (FDA).
HIGHLIGHTS:

- Clinical-stage biotechnology company: The Company is actively involved in developing cellular therapies for rare congenital life-threatening diseases and aging-related illnesses. It is now conducting clinical trials.
- Addressing unmet medical needs: LGVN is addressing critical unmet medical needs in conditions such as HLHS as well as aging-related conditions that include Alzheimer's disease, and aging-related frailty. The high demand for therapies in these areas indicates a significant potential market opportunity for Longeveron's products.
- Focus on aging-related conditions: LGVN focuses on addressing diseases and conditions associated with aging such as Alzheimer's disease, and aging-related frailty. The global market for Alzheimer's disease is projected to reach USD 18.46 Billion by 2029. While the global market for geriatric care services, including frailty management, is projected to reach over 1.6 Billion by 2028 driven by the increasing aging population and the need for specialized care.
- Growing Market Opportunities: The rise of the aging population and their demand for aging-related therapies offers promising opportunities, given that they constitute one of the world's largest and fastest-growing populations, - Additionally, there is an untapped opportunity in addressing rare diseases like HLHS. Today, there are approximately 7,000 known rare diseases with only 10% of them having an available medical treatment. Such an endeavor not only holds potential in terms of serving a critical unmet medical need but also presents a great potential commercial opportunity due to premium pricing for rare disease products and reduced operational costs due to low manufacturing volume.
- Lead product: The Company’s lead product is Lomecel-B™, derived from culture-expanded medicinal signaling cells (MSCs) sourced from the bone marrow of young, healthy adult donors. This product is being evaluated for various indications in multiple clinical trials.
- Clinical trial results: The Company recently announced the follow-up results from its Phase 1 Trial for Hypoplastic Left Heart Syndrome (HLHS) treating patients with Lomecel-B™ which demonstrated 100% (transplant-free) survival 3.5 – 5 years after treatment. In comparison, the Single Ventricle Reconstruction (SVR) Trial, the largest HLHS controlled trial to date, the survival rate was 80%. This gives the Company encouragement that Lomecel-B™ may potentially demonstrate long-term beneficial outcomes for these patients. -The Company anticipates a read-out of its Alzheimer's disease Phase 2a trial later this year.
- The Company’s aging-related frailty Phase 2b study showed a statistically significant increase in the 6-Minute Walk Test (“6MWT”) distance (a test of physical endurance - distance walked in 6 minutes) in multiple Lomecel-B™ treatment groups compared to placebo. Currently, a Phase 2 clinical trial in Japan is underway to assess the safety of Lomecel-B™ for aging-related frailty, with the potential for conditional approval based on efficacy and safety predictions.
- Potential for broad therapeutic application: LGVN believes that by utilizing MSCs, which may play a role in tissue repair, organ maintenance, and immune system function, it may be able to develop safe and effective therapies for challenging diseases and conditions associated with aging.
- Proprietary and scalable cellular therapy: LGVN has built a state-of-the-science in-house manufacturing facility to actively ensure its products are produced with the highest quality standards and follow strict cGMP guidelines.

Download Research Report
Longeveron Inc. (NASDAQ: LGVN) is a biotech company that is positioned to capitalize on serval opportunities in multi-billion dollar markets.
Hypoplastic Left Heart Syndrome (HLHS), Alzheimer's disease, and Aging Frailty represent pressing challenges with unmet medical needs
Hypoplastic Left Heart Syndrome (HLHS), a congenital heart defect primarily affecting infants, presents a complex and life-threatening condition. The Centers for Disease Control and Prevention (CDC) estimates that each year about 1,025 babies in the United States are born with hypoplastic left heart syndrome.
Despite advances in medical care, treatment options for HLHS have remained limited, resulting in high mortality rates. The need for novel regenerative solutions to address HLHS is paramount, both in terms of improving patient outcomes which, if successful, would then likely lead to that company/drug capturing a substantial market share in this underserved area.
An estimated 6.7 million Americans age 65 and older are living with Alzheimer’s Disease and dementia in 2023 and this number is projected to grow exponentially in the coming years. Unfortunately, there are extremely limited treatments available, leaving a significant unmet need for innovative therapies.
Another critical unmet need lies in the field of Aging Frailty. With the rapidly aging global population, the number of older adults facing frailty-related challenges is skyrocketing. Aging Frailty is associated with increased healthcare utilization and diminished quality of life. The demand for effective therapies to improve the health and independence of aging adults remains a critical need, creating a substantial opportunity for companies that are investing and conducting research in this area.
Longeveron seeks to be at the forefront of addressing unmet medical needs and leveraging regenerative medicine approaches to HLHS, Alzheimer's disease, and Aging Frailty.
With a strong commitment to scientific excellence and a mission to transform patient care, Longeveron Inc. (NASDAQ: LGVN) is working to make a significant impact in these critical areas and create substantial value in the market.
About the Company:
Longeveron Inc. is a clinical-stage biotechnology company that specializes in developing cellular therapies for aging-related and life-threatening conditions. The Company’s lead investigational product, Lomecel-B™, is derived from culture-expanded medicinal signaling cells sourced from the bone marrow of young healthy adult donors. By leveraging the regenerative properties of these cells, LGVN aims to create safe and effective therapies for challenging diseases and conditions associated with aging.
Company Mission: To advance the field of regenerative medicine, address unmet medical needs, and provide innovative treatments that improve patient outcomes and quality of life.
The Company is based in Miami, Florida, where its corporate headquarters and cGMP manufacturing facilities are located.
LOMECEL-B ™
Lomecel-B™ is Longeveron’s cellular therapy product being tested to address aging-related chronic diseases and other life-threatening conditions.
One of the key advantages of Lomecel-B™ is its superior efficacy in addressing inflammation. The therapy's medicinal signaling cells (MSCs) have the ability to migrate to sites of tissue damage. Additionally, Lomecel-B™ offers enhanced safety as it is inherently immuno-evasive, and its off-the-shelf administration further adds to its convenience.
Derived from MSCs isolated from donated bone marrow tissue, Lomecel-B™ is able to be administered without triggering an immune response or tissue rejection and is considered an “off-the-shelf” product due to the fact the cells can be administered on an outpatient basis in as little as 40 minutes after thawing, These characteristics give Lomecel-B™ an advantage over autologous cell therapy interventions, which require removing cells from the same individual and reintroducing them after weeks or months of culture.

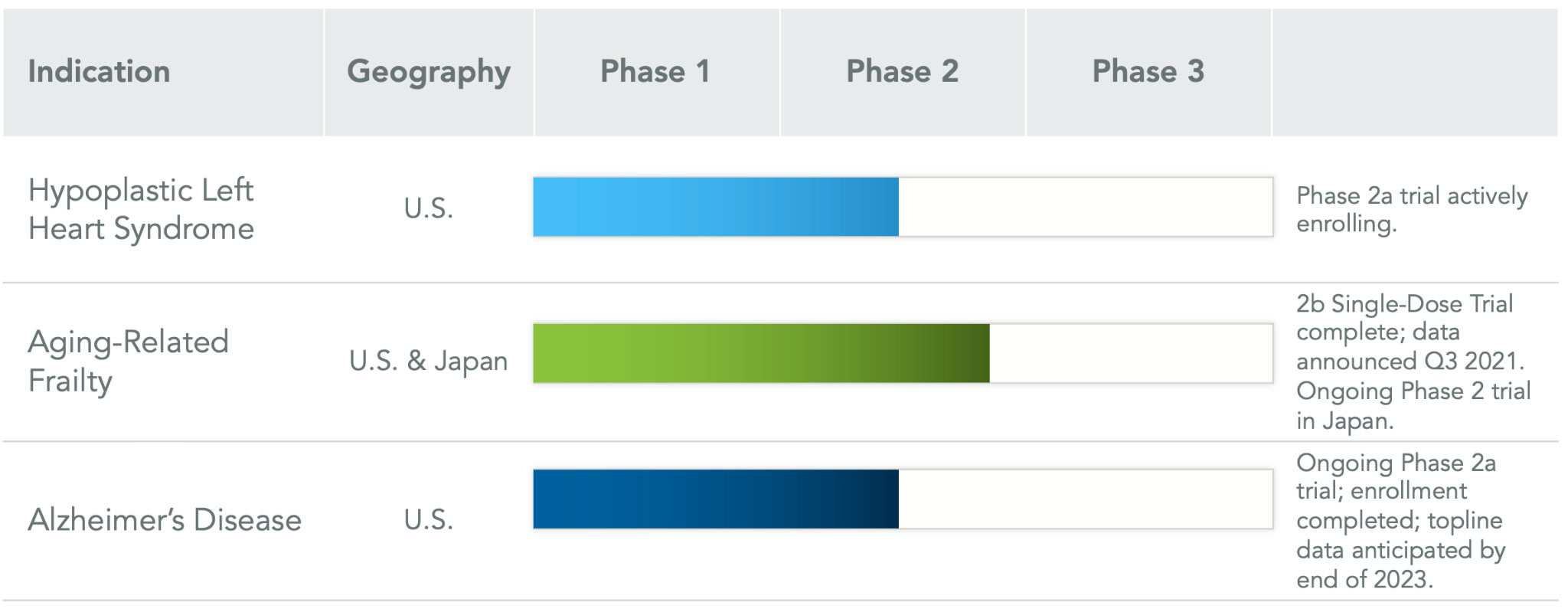
Hypoplastic Left Heart Syndrome (HLHS):
Lomecel-B™ is administered directly into the heart during Stage II surgery in HLHS patients. The primary endpoint is an improvement in Right Ventricular Ejection Fraction (RVEF), measured using MRI. Studies in adults have show that MSCs may increase ejection fraction and reduce ventricular chamber size.
The Phase 1b trial (ELPIS 1) of Lomecel-B™ in HLHS demonstrated positive results, with no serious adverse events reported, no need for heart transplants among the treated patients during the one-year study duration, and Longeveron was granted rare pediatric disease designation, orphan drug designation, and fast track designation from the FDA.
The ongoing Phase 2 trial (ELPIS II) aims to evaluate the efficacy of Lomecel-B™ in HLHS patients through a randomized, controlled study with a total population of 38 patients. The primary endpoint of this trial is the change in Right Ventricular Ejection Fraction (RVEF) at 12 months.
Alzheimer’s Disease:
Lomecel-B™ is being investigated as a potential treatment for Alzheimer's disease (AD), focusing on targeting central nervous system inflammation. Previous therapies targeting amyloid plaques or neurofibrillary tangles have shown limited improvement. Inflammation is increasingly recognized as a key potential pathway in AD pathogenesis. Animal models have shown that MSCs can cross the blood-brain barrier, reduce inflammation, improve immune functioning, promote neurogenesis, and enhance endothelial function.
In the Phase 1b trial involving mild AD patients, a single infusion of Lomecel-B™ demonstrated safety, with no serious adverse events attributed to the treatment. The low-dose Lomecel-B™ group showed statistically significant cognitive improvement compared to placebo and high-dose groups, as measured by Mini-Mental State Exam (MMSE) scores. Quality of life also appeared to improve in the low-dose group.
A Phase 2a study is currently ongoing, exploring multiple doses of Lomecel-B™ in mild AD patients, with topline data from this Phase 2a Alzheimer’s Disease trial anticipated by the end of 2023.
Aging-Related Frailty:
Lomecel-B™ is being investigated as a potential treatment for aging-related frailty, a condition characterized by a decline in multiple physiological systems and increased vulnerability to stressors.
The Phase 2b study included 143 participants aged 70 to 85 with evidence of inflammation and mild to moderate frailty. The primary endpoint was the 6-minute walk test (6MWT), a measure of physical endurance. Results showed a statistically significant increase in 6MWT distance in multiple Lomecel-B™ treatment groups at nine (9) months, compared to placebo. Adverse events were primarily related to the administration process.
In Japan, a clinical trial is underway to assess the safety of Lomecel-B™ for aging-related frailty, with the potential for conditional approval based on efficacy and safety predictions.
Leadership:

Wa’el Hashad – Chief Executive Officer
Wa’el Hashad joined Longeveron in the role of CEO on March 1, 2023, bringing with him more than 35 years of experience in the pharmaceutical and biotech industries.
Prior to joining Longeveron, Mr. Hashad was the President and CEO of Avanir Pharmaceuticals where he led all aspects of the company’s commercial initiatives and the product development pipeline. Avanir was fully integrated into Otsuka on December 22, 2022. In his career before Avanir, Mr. Hashad was the Chief Commercial Officer of Seres Therapeutics, where he spearheaded both strategy and development of various therapeutic types, including several microbiome-based therapies. Mr. Hashad is an accomplished leader, who has held senior leadership positions at Amgen, Boehringer Ingelheim and Eli Lilly and Company.
He has launched several successful brands in the U.S. and worldwide markets. His therapeutic expertise includes cardiovascular, neuroscience, endocrine and inflammatory diseases. He is passionate about innovation and advancing science. Mr. Hashad holds a Bachelor of Science in Pharmacy from Cairo University and a Master of Business Administration from University of Akron in Ohio.

Joshua M. Hare, M.D. – Co-Founder, Chief Science Officer and Chairman
Dr. Hare co-founded Longeveron in 2014 and serves as Chairman of the Board of Directors, and Chief Science Officer. Dr. Hare is a double board-certified cardiologist (Cardiology and Advanced Heart Failure and Transplantation) and is the founding director of the Interdisciplinary Stem Cell Institute at the University of Miami’s Miller School of Medicine.
He has obtained in excess of $25 Million in funding from the National Institutes of Health over the past 15 years to support basic research of cell therapy strategies.
He is also a recipient of the Paul Beeson Physician Faculty Scholar in Aging Research Award, and is an elected member of the American Association of Physicians, The American Society for Clinical Investigation, and is an elected Fellow of the American Heart Association.
Dr. Hare has also served in numerous leadership roles at the American Heart Association and at the Center for Scientific Review of the National Institutes of Health. Dr. Hare is also a co-founder of Vestion, Inc., and Heart Genomics, LLC, companies that hold cardio-related intellectual property.
He received a BA from the University of Pennsylvania, and his MD from The Johns Hopkins University School of Medicine, and completed fellowships at Johns Hopkins and Brigham and Women’s Hospital, and was a Research Fellow at Harvard Medical School.

Lisa Locklear – Chief Financial Officer
Ms. Locklear joined Longeveron in the role of CFO on July 31, 2023. Before Longeveron, Lisa served as the Senior Vice President and CFO for Avanir Pharmaceuticals, a subsidiary of Otsuka. During her time at Avanir, Ms. Locklear was instrumental in enhancing the financial and technology-related processes, systems, and people during a period of rapid growth. Prior to Avanir, she held senior financial roles at GSN Games, CoreLogic, Ingram Micro, the Walt Disney Company, and Price Waterhouse (now PwC), with assignments in Paris and London.
Ms. Locklear has been recognized by the Healthcare Businesswomen’s Association with the Luminary Award, an honor that underscores her dedication to fostering the growth of other women’s careers and her unwavering commitment to the healthcare industry.
In addition to her professional career, Ms. Locklear serves on several philanthropic boards. She currently chairs the Board of Governors for the Gemological Institute of America (GIA), and serves on the boards of the Pacific Marine Mammal Center and the Orange County United Way, and is a member of the National Association of Corporate Directors (NACD).
Ms. Locklear holds a B.S. in plant science from the University of California, Davis, and an MBA from the University of California, Irvine. She is a licensed CPA (inactive) and is a member of the American Institute of Certified Public Accountants, the California Society of CPAs, and Financial Executives International.

Nataliya Agafonova, M.D. – Chief Medical Officer
Dr. Agafonova joined Longeveron in the role of CMO on July 1, 2023. Before Longeveron, she served as Clinical Development Lead, Senior Medical Director, and Product Development Chair at Otsuka Pharmaceuticals. Previously, she was the Clinical Development Lead and Senior Medical Director at Bristol-Myers Squibb. Dr. Agafonova previously held several senior leadership positions in clinical development and pharmacovigilance at Ardea Bioscience, Biogen, Amgen, and Genzyme Corporation.
Dr. Agafonova has extensive experience in therapeutic areas such as autoimmune, hematology, neuroscience, and oncology. Her cross-therapeutic expertise in drug development helped to bring several products to the U.S. and EU markets.
Prior to her industry experience, Dr. Agafonova served as a physician at the Ukrainian Research Institute of Oncology and Radiology.
Dr. Agafonova earned an M.D. from the Ukrainian National Medical University and completed her internal medicine residency at Kharkov State University Hospital in Ukraine.

Paul Lehr – General Counsel and Secretary
Paul Lehr joined Longeveron in 2016 and serves as General Counsel and Corporate Secretary. Over the past 25 years, Mr. Lehr has held senior legal and executive positions in corporate, non-profit, and research settings.
Mr. Lehr started his legal career as a law clerk for a United States Federal Judge and thereafter practiced law at a leading Miami law firm for 5 years, with experience in healthcare and business. Thereafter, Mr. Lehr focused his efforts in the cardiac rehabilitation field as President and General Counsel of a non-profit research foundation and for-profit cardiac rehabilitation program. With the research serving as the foundation of the for-profit arm of the cardiac rehabilitation program, Mr. Lehr negotiated a master franchise agreement with a leading Indian healthcare operator with 100+ facilities across India and the Middle East, then co-lead negotiations with the Centers for Medicare & Medicaid Services to successfully secure CMS reimbursement of their intensive cardiac rehabilitation program.
Mr. Lehr has also served since 2011 as CEO and co-founder of Heart Genomics, LLC, a biotech firm based on intellectual property that Mr. Lehr licensed from the UM Miller School of Medicine.
Mr. Lehr earned his B.A. from Brown University and his J.D. with honors from the University of Florida College of Law.
In Conclusion…
Longeveron Inc. (NASDAQ: LGVN) is a clinical-stage biotech company that deserves attention due to its focus on addressing unmet medical needs in regenerative diseases.
With a mission to deliver regenerative medical therapies, LGVN is at the forefront of developing innovative treatments for conditions such as Hypoplastic Left Heart Syndrome (HLHS), Alzheimer's disease, and aging-related frailty.
Longeveron's focus on aging-related conditions aligns with the growing global demand for therapies in this area. The market for Alzheimer's disease and geriatric care services is projected to reach billions of dollars, driven by an increasingly aging population and the need for specialized care.
The Company's clinical pipeline shows promising results, with positive outcomes in Phase 1 trials for various indications.
As a clinical-stage company, Longeveron has progressed beyond the initial stages and is actively conducting clinical trials, positioning itself for future growth and success.
With a commitment to scientific excellence and a mission to transform patient care, Longeveron Inc. (NASDAQ: LGVN) has the potential to make a significant impact in addressing unmet medical needs and capturing value in the regenerative medicine market.

THIS IS A PAID ADVERTISEMENT
NO INVESTMENT ADVICE
SCD Media LLC (d/b/a “Smallcaps Daily”), hereinafter referred to as “Smallcaps Daily,” and their affiliates and control persons (the “Publisher”) are in the business of publishing favorable information and/or advertisements (the “Information”) about the securities of publicly traded companies (each an “Issuer” or collectively the “Issuers”) in exchange for compensation (the “Campaigns”). Persons receiving the Information are referred to as the “Recipients.” The person or entity paying the Publisher for the Campaign is referred to herein as the “Paying Party”. The Paying Party may be an Issuer, an affiliated or non-affiliate shareholder of an Issuer, or another person hired by the Issuer or an affiliate or non-affiliate shareholder of the Issuer. The nature and amount of compensation paid to the Publisher for the Campaign and creating and/or publishing the Information about each Issuer is set forth below under the heading captioned, “Compensation”.
This website provides information about the stock market and other investments. This website does not provide investment advice and should not be used as a replacement for investment advice from a qualified professional. This website is for informational purposes only. The Author of this website is not a registered investment advisor and does not offer investment advice. You, the reader, bear responsibility for your own investment decisions and should seek the advice of a qualified securities professional before making any investment.
Nothing on this website should be considered personalized financial advice. Any investments recommended herein should be made only after consulting with your personal investment advisor and only after performing your own research and due diligence, including reviewing the prospectus or financial statements of the issuer of any security.
Smallcaps Daily, its managers, its employees, affiliates, and assigns (collectively the "Publisher") do not make any guarantee or warranty about the advice provided on this website or what is otherwise advertised above.
Release of Liability: through use of this website, viewing or using you agree to hold Smallcaps Daily, its operators, owners, and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources that we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. Smallcaps Daily encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the company profiled or is available from public sources and Smallcaps Daily makes no representations, warranties, or guarantees as to the accuracy or completeness of the disclosure by the profiled company. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provided herein. Instead, Smallcaps Daily strongly urges you to conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Smallcaps Daily’s full disclosure is to be read and fully understood before using Smallcaps Daily's website, or joining Smallcaps Daily's email or text list. From time to time, Smallcaps Daily will disseminate information about a company via website, email, sms, and other points of media. By viewing Smallcaps Daily's website and/or reading Smallcaps Daily's email or text newsletter you are agreeing to this ----> https://Smallcaps Daily.com/disclaimer/. All potential percentage gains discussed in any communications are based on calculations from the low to the high of the day. We are engaged in the business of marketing and advertising companies for monetary compensation.
If you have questions or concerns about a product you’ve seen in one of our emails, emails, text newsletters or SMS, we encourage you to reach out to that company directly.
Disclaimer – Always do your own research and consult with a licensed investment professional before investing. This communication is never to be used as the basis of making investment decisions and is for entertainment purposes only. At most, this communication should serve only as a starting point to do your own research and consult with a licensed professional regarding the companies profiled and discussed. Conduct your own research. This newsletter is a paid advertisement, not a recommendation nor an offer to buy or sell securities. This newsletter is owned, operated, and edited by the owner of Smallcaps Daily. Any wording found in this e-mail or disclaimer referencing to “I” or “we” or “our” refers to Smallcaps Daily. Our business model is to be financially compensated to market and promote small public companies. By reading our newsletter and our website you agree to the terms of our disclaimer, which are subject to change at any time. We are not registered or licensed in any jurisdiction whatsoever to provide investing advice or anything of an advisory or consultancy nature and are therefore unqualified to give investment recommendations. Companies with low prices per share are speculative and carry a high degree of risk, so only invest what you can afford to lose. By using our service, you agree not to hold our site, its editors, owners, or staff liable for any damages, financial or otherwise, that may occur due to any action you may take based on the information contained within our newsletters or on our website. We do not advise any reader to take any specific action. Losses can be larger than expected if the company experiences any problems with liquidity or wide spreads. Our website and newsletter are for entertainment purposes only. Never invest purely based on our alerts. Gains mentioned in our newsletter and on our website may be based on end-of-day or intraday data. This publication and its owners and affiliates may hold positions in the securities mentioned in our alerts, which we may sell at any time without notice to our subscribers, which may have a negative impact on share prices. If we own any shares, we will list the information relevant to the stock and the number of shares here.
COMPENSATION
In compliance with section 17(b) of the Securities Act we are disclosing that we have been compensated a fee pursuant to an agreement between Smallcaps Daily and TraDigital Marketing Group, Inc. (d/b/a/ “TraDigital IR”) hereinafter referred to as TraDigital IR. Please see TraDigital IR’s disclosure page here. Smallcaps Daily was hired by TraDigital IR for a period beginning April 2021 and ending October 2021 to publicly disseminate information about Longeveron Inc. via website, email, and SMS. We were paid five thousand USD via ACH. Readers are advised to review SEC periodic reports: forms 10Q 10K, form 8K, insider reports, forms 3, 4, 5 schedule 13d. Smallcaps Daily is compliant with the CAN-SPAM Act of 2003. Smallcaps Daily does not offer investment advice or analysis, and Smallcaps Daily further urges you to consult your own independent tax, business, financial, and investment advisors. investing in micro-cap, small-cap, and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor's investment may be lost or impaired due to the speculative nature of the companies profiled. The private securities litigation reform act of 1995 provides investors a safe harbor in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events, or performance are not statements of historical fact but may be forward-looking statements. Forward-looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward-looking statements in this action may be identified through the use of words such as projects, foresee, expects, will, anticipates, estimates, believes, understands, or that by statements indicating certain actions & quotes; may, could, or might occur. Understand there is no guarantee past performance will be indicative of future results in preparing this publication. Smallcaps Daily has relied upon information supplied by its clients, as well as its clients’ publicly available information and press releases which it believes to be reliable; however, such reliability can not be guaranteed. Investors should not rely on the information contained on this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, Smallcaps Daily and its owners, affiliates, subsidiaries, officers, directors, representatives, and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of material facts from such advertisement. Smallcaps Daily is not responsible for any claims made by the companies advertised herein, nor is Smallcaps Daily responsible for any other promotional firm, its program, or its structure. Smallcaps Daily is not affiliated with any exchange, electronic quotation system, the Securities Exchange Commission, or FINRA.
Longeveron Inc. is a client of TraDigital IR, an investor relations and communications firm. Please see TraDigital’s disclosures at www.tradigitalir.com.
Copyright © 2022 Smallcaps Daily. All rights reserved.