With its flagship diagnostics test ColoAlert, this little-known NASDAQ company makes early detection of colon cancer easier than ever….
As cases of colorectal cancer continue to affect people, with now younger groups being diagnosed, Mainz Biomed N.V. (NASDAQ: MYNZ) may represent one of the biggest health stock opportunities hiding in the market!
Despite significant progress, cancer continues to be the second-largest cause of death in the U.S. Unfortunately, more than 1.9 million new cases of cancer are likely to be diagnosed in the U.S. alone this year and more than 600,000 deaths are expected from the disease.
This is a good time to take a look at the cancer-fighting space on Wall Street, especially as healthcare is often considered an inflation hedge.
Download Research Report
10 Immediate reasons to be paying attention to MYNZ
- A flagship product that could position company as a leader in early detection for colorectal cancer.
- Former executives from major pharma giants on board… the company’s CEO Guido Baechler was a former Roche Molecular diagnostics executive… Chief Commercial Officer Darin Leigh held executive commercial positions at several leading life science and healthcare firms including Abbott, Luminex, Metabolon, and CDR Health!;
- Exciting FDA feedback has been received for their lead product ColoAlert...
- A whopping $25 price target….
- ColoAlert rivals Cologuard from Exact Sciences Corporation (NASDAQ: EXAS) which trades over $60 a share…
- Ramped up international commercial activities for ColoAlert, with at least five new lab partners in Germany and Italy…
- Ample cash to fund operations…
- Executed its option from Uni Targeting Research AS to acquire all of the previously licensed scientific intellectual property ("I.P.") for its flagship product ColoAlert. That deal makes ColoAlert a wholly-owned asset…
- Exercised its exclusive option with SOCPRA Sciences Sante et Humaines S.E.C. to outright purchase I.P., including a pending patent, associated with a portfolio of novel gene expression (mRNA) biomarkers that have demonstrated the ability to detect C.R.C. lesions, including advanced adenomas, a type of pre-cancerous polyp often attributed to this deadly disease…
- A commercial strategy and product development plans intended to bring what the company describes as the gold-standard C.R.C. self-administered diagnostic test to market…
Colorectal cancer is the third most common cancer globally, with more than 1.9 million new cases in 2020 alone. There is a dire need for early detection!

“Our goal is to reduce these statistics and save lives by raising awareness about the simplicity and efficacy of using at-home CRC detection kits, like ColoAlert. Our non-invasive DNA stool test allows people to screen themselves in the comfort of their own home and then receive a follow-up notification from their physician to discuss results and next steps.”
Guido Baechler, Chief Executive Officer of Mainz Biomed
ColoAlert Holds Potential as a Blockbuster Early Detection Test for Colorectal Cancer and Could Turn Mainz Biomed (NASDAQ: MYNZ) into a 2023 Breakout Story!
Greetings Investors,
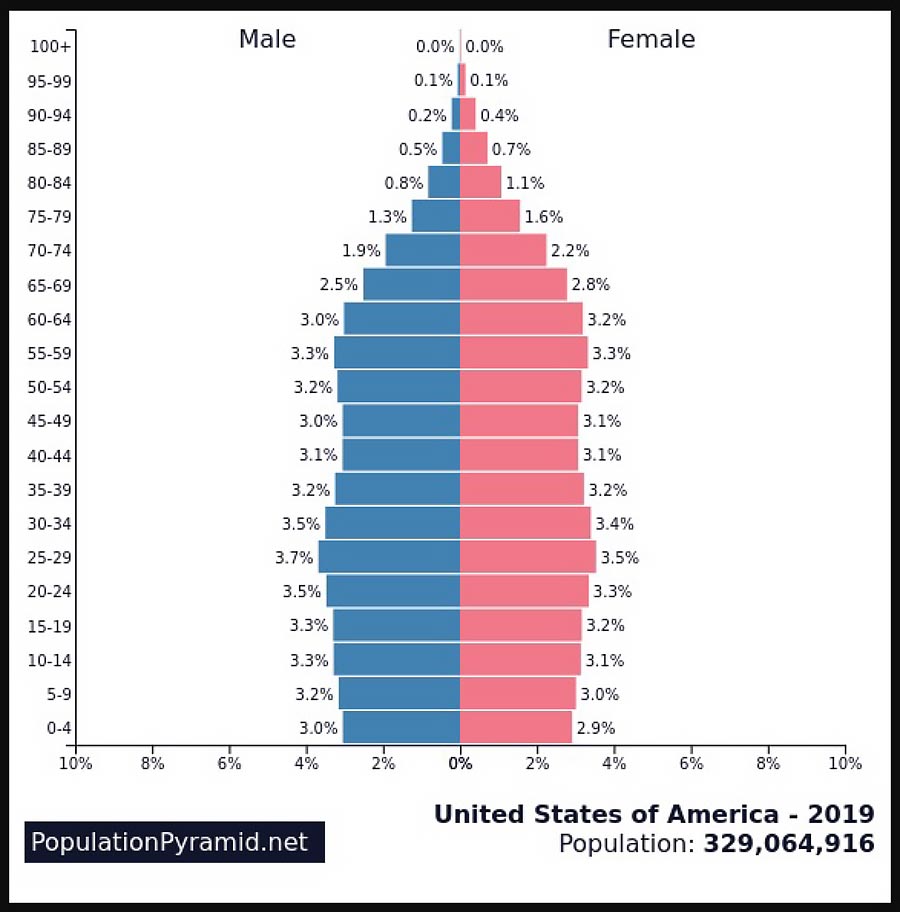
Overall, the lifetime risk of developing colorectal cancer is about 1 in 23 for men and 1 in 26 for women says the American Cancer Society.
The American Cancer Society’s estimates for the number of colorectal cancers in the United States for 2023 are:
- 106,970 new cases of colon cancer
- 46,050 new cases of rectal cancer
And colorectal cancer cases are rising in young adults.
Why is there an uptick? Nobody knows for sure but a sedentary lifestyle, overweight and obesity, smoking, heavy alcohol use, low-fiber, high-fat diets or diets high in processed meats, and other environmental factors have all been associated with the disease.
Screening is important because when found early, colorectal cancer is highly treatable. Early stages of colorectal cancer usually present no symptoms so screening is crucial.
According to the Centers for Disease Control and Prevention (“CDC”), Colorectal cancer (CRC) is the second most lethal cancer in the United States and Europe, but also the most preventable, with early detection providing survival rates above 90%!
A colonoscopy is one of several screening tests for colorectal cancer, but they can be awfully invasive.
MYNZ’s flagship product is ColoAlert, an accurate, non-invasive, and easy-to-use early detection diagnostic test for colorectal cancer. It is simple, fast, accurate, and non-invasive.
ColoAlert detects colorectal cancer (CRC) via a simple-to-administer test with a sensitivity and specificity nearly as high as the invasive colonoscopy (Dollinger MM et al., 2018). The test utilizes proprietary methods to analyze cell DNA for specific tumor markers combined with the fecal immunochemical test (FIT). It is designed to detect tumor DNA and CRC cases in their earliest stages.


Patients receive a simple kit that includes instructions, a stool collector and shipping instructions to return the kit through regular mail to their local lab for testing and results. IT’S THAT EASY.
While the product is not approved yet for the U.S., it may be in the future, and this could be a game changer considering that it needs smaller samples than rival Cologuard!
ColoAlert Highlights
- A PCR-based molecular genetic CRC early detection stool test
- Up to 60% fewer missed cases compared to fecal immunochemical test (FIT) 10
- Non-invasive, no preparation or sedation, no time off work
- 98% patient satisfaction – Easy product to use 11
- Designed to offer affordable CRC screening solutions
10 Comparing ColoAlert sensitivity with FITs (Gies et al. Gastroenterology 154/2018)
11 98% overall satisfaction with ColoAlert in our internal customer survey.
PROPRIETARY STATE OF THE ART TECHNOLOGY
- Colorectal cancer begins when cells in the intestine undergo genetic mutations. Tumor cells shed into the intestines. Stool samples can be examined for tumor DNA using PCR technology.
- Polymerase chain reaction (PCR) is used to rapidly make millions to billions of copies of a specific DNA sample and only requires a very small sample of DNA to amplify it large enough for detailed study.
- ColoAlert utilizes proprietary methods to analyse the cell DNA for specific tumor markers.
- ColoAlert is designed to detect tumor DNA and detect 85% of colorectal cancer cases - often in the earliest stages of the disease.*
- Ease-of-use drives ongoing patient adherence as the ColoAlert method requires very small samples compared to ColoGuard.
*Dollinger MM et al. (2018), ClinLab 64 (10), 1719-1730 and Gies et al. (2018). Gastroenterology 154 (1), 93-104.and Cooper GS et al. (2018). Dig Dis Sci. 63 (6), 1449-1453. * 18 study centres, 566 patients, 10/2018 and Amani et al. (2019). Clin. Lab. 65:1751-1754.
COMPANY OVERVIEW
Mainz Biomed develops market-ready molecular genetic diagnostic solutions for life-threatening conditions and is transforming at-home cancer detection.
Clinical laboratory tests save costs and lives by enabling early detection and prevention of disease. Patients with cancers and other conditions are living longer and enjoying better health because of medical revolutions in diagnostic technology.
At its center are genetic and genomic tests that identify the unique genetic profile of individual patients or their disease and allow physicians to tailor treatment to those unique characteristics.
Key Corporate & Product Development Highlights in 2022 include:
- Accelerated international commercial activities for ColoAlert, the Company’s highly efficacious and easy-to-use detection test for colorectal cancer (CRC).
- Appointed Darin Leigh, former Abbott and Luminex executive, as Chief Commercial Officer.
- Established partnership with Dante Labs to market ColoAlert in Italy and the United Arab Emirates.
- A high-profile partnerships with leading laboratory Labor MVZ Dr. Stein + Kollegen, commonly referred to as “Laboratory Mönchengladbach” (February), one of the largest diagnostics laboratories in Germany, servicing over 2,500 physicians, processing over five million samples annually and screening approximately 1,000 patients per week specifically for CRC.
- Initiated and enrolled the first patient in an international clinical study (ColoFuture) evaluating the integration of novel mRNA biomarkers into ColoAlert – potentially upgrading its technical profile to achieve “gold standard” status for CRC at-home testing.
- Received supportive feedback from the U.S. Food and Drug Administration (FDA) on ColoAlert’s pre-submission package for its U.S. pivotal clinical trial set to commence in Q4 2022.
- Achieved multiple preclinical milestones supporting the continued development of PancAlert, a potential first-in-class screening test for pancreatic cancer.
- Executed a $25.8 million (gross) public follow-on offering.
- Expanded Strategic Advisory Board of global leaders in molecular diagnostic development and commercialization.

COMMERCIALIZATION ACROSS EUROPE AND INTERNATIONAL MARKETS
Mainz Biomed is commercializing ColoAlert across Europe and in select international markets through a differentiated business model of partnering with third-party laboratories for test kit processing versus the traditional methodology of operating a single facility.
Under the standard terms of all partnerships, Mainz Biomed is providing ColoAlert to the respective labs, including co-branding with key accounts, whereby each facility purchases Mainz Biomed’s customized polymerase chain reaction (“PCR”) assay kits on an on-demand basis and provides their respective network of physicians and patients with a comprehensive solution for advanced CRC detection.

A STRONG PARTNERSHIP FOR GROWTH
DNA extraction for the company’s flagship product ColoAlert is now automated on the Thermo Scientific™ KingFisher™ Apex.
This essential partnership enables laboratories around the world to increase their testing capacity and to optimize their resource allocation.
- Automated DNA extraction with magnetic particles
- Tailored protocol for ColoAlert
- Up to 96 samples per run in ~ 2 hours*
- Brings convenience, performance and reproducibility to the ColoAlert workflow
* Extraction time, does not include sample pre-treatment. For Laboratory Use.
ENHANCING ALREADY EXCELLENT ASSETS
MYNZ is evaluating the mRNA biomarkers acquired from SOCPRA in ColoFuture and eAArly DETECT, an international multi-center clinical study (U.S. and Europe) assessing the potential for integrating the mRNA biomarkers into ColoAlert.
This particular portfolio of mRNA biomarkers selected by the company was based on work in the field by the University of Sherbrooke, where researchers tested multiple novel transcriptional biomarkers using colorectal cancer and pre-cancerous lesion samples.
Results from these studies demonstrated that the mRNA targets chosen by the company provided a dynamic combination of sensitivity and specificity of detection.

The ColoFuture study (extended into the U.S. as eAArly DETECT) is evaluating the effectiveness of these biomarkers to enhance ColoAlert's technical profile to expand its capability to identify A.A. while increasing ColoAlert's rates of diagnostic sensitivity and specificity. As noted, ColoFuture's eAArly DETECT study is on track to complete enrollment in the first quarter of 2023, with results also expected in the first half of 2023. That milestone, once reached, could become a catalyst for growth!
Even studying mRNA biomarkers is a hot sector. Both Pfizer (NYSE: P.F.E.) and Moderna (NASDAQ: MRNA) are investing considerable resources in appraising their value in a new generation of medicine!
MYNZ has highlighted that the outcome of its eAArly DETECT study will inform its decision of whether to integrate the biomarkers into the ReconAAsense study, which is on track to enroll patients in the summer of 2023!
The results from that study are planned to be reported in 2025, forming the basis of the data package to be reviewed by the F.D.A. to achieve marketing authorization.
MYNZ has also announced additional commercial partnerships for ColoAlert with Marylebone Laboratory and Instituto de Microecologia, two leading independent laboratories covering England and Spain.
That deal will expand MYNZ's revenue-generating reach and target an addressable market in Spain estimated at 26 million patients and a London-region patient treatment opportunity of roughly 9 million individuals.
Revenues from that deal could accrue quickly. MYNZ says they are working on completing the necessary technical and co-marketing activities to ensure a successful commercial launch in these markets.
COMMERCIAL PIPELINE DEVELOPMENT: FUTURE PRODUCTS
In addition to MYNZ's flagship ColoAlert product, the company is also developing a novel early-detection pancreatic cancer screening test.
Mainz BioMed is currently developing proprietary genetic testing methods for pancreatic cancer.
- Fighting what could soon become the world’s second most deadly cancer.
- Convenient stool test for at-home use.
- Potential for over 50 million tests per year in Europe alone
- Supported by federal grant from Germany’s Federal Ministry for Education and Research.
- Cost of goods sold (COGS) & reimbursement analogous to ColoAlert program.

PRIORITY ROLLOUT MARKETS
- ColoAlert launches from Germany… to Europe and America
- Expansion into EU markets is aligned with early-stage plans for American market entry.
- Upon FDA approval, Mainz BioMed plans to offer ColoAlert CRC screening test kits to national reference labs and major health institutions in the USA.
- Mainz BioMed is carefully evaluating FDA requirements to ensure an expedited strategy is aligned with future clinical, regulatory and related guideline requirements.
- Key clinical studies will be co-located in the US and abroad to meet the requirements set by FDA for a screening application
IN SUMMARY
With every problem comes an opportunity -- in this case, a massive opportunity because cancer is a big problem. Both large and small companies are developing new ways of diagnosing and treating cancer and MYNZ may have one of the best ways to fight off colon cancer early on.
The company's closest peer, Exact Sciences (NASDAQ: EXAS) - ColoGuard product is only sold in the US - and has a market cap of about $11 billion. TO REITERATE.... COLOALERT IS EASIER TO ADMINISTER AND MORE ACCURATE!
AND… MYNZ currently carries an $18 price target! With leading analysts CANTOR FITZGERALD and H C WAINWRIGHT recommending the stock!
With a blockbuster early detection test for colorectal cancer that could give Exact Science's Cologuard a run for its money... now is the time to be paying attention to emerging player Mainz Biomed N.V. (NASDAQ: MYNZ)!
Learn More about Mainz Biomed N.V. by gaining access to the latest
research report
Download


THIS IS A PAID ADVERTISEMENT
NO INVESTMENT ADVICE
Copyright 2022 © SCDalerts.com is owned and operated by the owner of SCD Media LLC.
Disclaimer and Privacy For more Information please contact info@smallcapsdaily.com
This website provides information about the stock market and other investments. This website does not provide investment advice and should not be used as a replacement for investment advice from a qualified professional. This website is for informational purposes only. The Author of this website is not a registered investment advisor and does not offer investment advice. You, the reader, bear responsibility for your own investment decisions and should seek the advice of a qualified securities professional before making any investment. Nothing on this website should be considered personalized financial advice. Any investments recommended here in should be made only after consulting with your personal investment advisor and only after performing your own research and due diligence, including reviewing the prospectus or financial statements of the issuer of any security.
SCD Media, its managers, its employees, affiliates, and assigns (collectively "The Company") do not make any guarantee or warranty about the advice provided on this website or what is otherwise advertised above. To the maximum extent permitted by law, the Company disclaims all liability in the event any information, commentary, analysis, opinions, advice and/or recommendations provided herein prove to be inaccurate, incomplete, or unreliable, or result in any investment or other losses.
You received this message as part of your subscription to SCD Alerts.
SCD Alerts is a financial news and information website. We do not directly sell any products or offer any personal financial advice, nor do we advocate the purchase or sale of any security or investment for any specific individual. We also do not make any guarantee or warranty about what is advertised above.
If you have questions or concerns about a product you’ve seen in one of our emails, we encourage you to reach out to that company directly. Disclaimer – Always do your own research and consult with a licensed investment professional before investing. This communication is never to be used as the basis of making investment decisions and is for entertainment purposes only. At most, this communication should serve only as a starting point to do your own research and consult with a licensed professional regarding the companies profiled and discussed. Conduct your own research. This newsletter is a paid advertisement, not a recommendation nor an offer to buy or sell securities. This newsletter is owned, operated, and edited by SCD Media. Any wording found in this e-mail or disclaimer referencing “I” or “we” or “our” or “SCD” refers to SCD Media. Our business model is to be financially compensated to market and promote small public companies. By reading our newsletter and our website you agree to the terms of our disclaimer, which are subject to change at any time. We are not registered or licensed in any jurisdiction whatsoever to provide investing advice or anything of an advisory or consultancy nature and are therefore unqualified to give investment recommendations. Companies with low prices per share are speculative and carry a high degree of risk, so only invest what you can afford to lose. By using our service, you agree not to hold our site, its editor’s, owners, or staff liable for any damages, financial or otherwise, that may occur due to any action you may take based on the information contained within our newsletters or on our website. We do not advise any reader to take any specific action. Losses can be larger than expected if the company experiences any problems with liquidity or wide spreads. Our website and newsletter are for entertainment purposes only.Never invest purely based on our alerts. Gains mentioned in our newsletter and on our website may be based on end-of-day or intraday data. This publication and its owners and affiliates may hold positions in the securities mentioned in our alerts, which we may sell at any time without notice to our subscribers, which may have a negative impact on share prices. If we own any shares, we will list the information relevant to the stock and the number of shares here.
We do not own any shares in MYNZ. We have been currently compensated Fifteen Thousand Dollars Cash ($15,000) via bank wire transfer from a third-party IA Media, LLC for a 1 Day Marketing Program regarding MYNZ with a start date of 3/20/2023. SCD’s business model is to receive financial compensation to promote public companies. This compensation is a major conflict of interest in our ability to be unbiased regarding our alerts. Therefore, this communication should be viewed as a commercial advertisement only. We have not investigated the background of the hiring third party or parties. The third party, profiled company, or their affiliates likely wish to liquidate shares of the profiled company at or near the time you receive this communication, which has the potential to hurt share prices. Any non- compensated alerts are purely for the purpose of expanding our database for the benefit of our future financially compensated investor relations efforts.
Frequently companies profiled in our alerts may experience a large increase in volume and share price during investor relations marketing, which may end as soon as the investor relations marketing ceases. The investor relations marketing may be as brief as one day, after which a large decrease in volume and share price is likely to occur. Our emails may contain forward looking statements, which are not guaranteed to materialize due to a variety of factors. We do not guarantee the timeliness, accuracy, or completeness of the information on our site or in our newsletters. The information in our email newsletters and on our website is believed to be accurate and correct but has not been independently verified and is not guaranteed to be correct. The information is collected from public sources, such as the profiled company’s website and press releases, but is not researched or verified in any way whatsoever to ensure the publicly available information is correct.
Furthermore, SCD often employs independent contractor writers who may make errors when researching information and preparing these communications regarding profiled companies. Independent writers’ works are double-checked and verified before publication, but it is certainly possible for errors or omissions to take place during editing of independent contractor writer’s communications regarding the profiled company(s). You should assume all information in all of our communications is incorrect until you personally verify the information, and again are encouraged to never invest based on the information contained in our written communications.
The information in our disclaimers is subject to change at any time without notice.