This Quietly Trading NASDAQ Company Has a Unique Technology Aimed to Combat Critical Health Conditions Including Obesity and Type 2 Diabetes.

Healthcare is undergoing a digital revolution.
Big Data, mobile devices and other innovations are opening up new frontiers in medicine, a trend that has become especially visible during the COVID-19 pandemic.
Looking beyond the pandemic, digitalization in healthcare is expected to improve a broad range of outcomes, from the prevention and treatment of disease to nursing care.
Founded in 2011, NMRD set out to develop a single platform technology using non-invasive microsystems to measure blood markers at the surface of the skin.
Since then, the company has evolved with the creation of wearable technologies and digital healthcare solutions that encourage and empower people to take charge of their own health and wellbeing.
The company is replacing traditional invasive methods of diagnosis and healthcare observation procedures with its BEAT® technology platform that will allow for remote continuous monitoring of chronic diseases and health conditions without using needles.
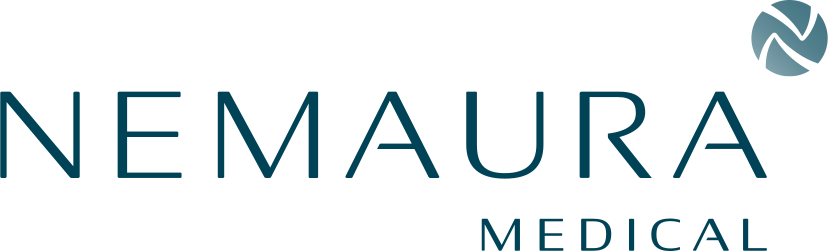
Download Research Report
NMRD recently announced that it has received its first purchase order from U.S. health provider HealthFleet, a leading telehealth provider that focuses on care, coaching, and health recommendations.
"The HealthFleet purchase order is our first collaboration in the United States and provides an important opportunity to potentially improve outcomes for people with type 2 diabetes by integrating our proBEAT platform into HealthFleet’s RestoreHealth program. We are confident that our proprietary sensor technology can improve patient engagement and potential outcomes in the RestoreHealth program, and believe that the improved outcomes to be seen in this pilot can help to position this as the program of choice across the U.S. health economy."
Dr. Faz Chowdhury, Nemaura’s CEO
The purchase order consists of 75,000 proBEAT glucose sensors over an initial five-month period and is valued at $500,000 in revenue. The order may be the first of many more to come as NMRD leverages its highly innovative BEAT® technology!
Greetings Investors,
The last couple of years have profoundly highlighted the importance of health. The pandemic sadly revealed the consequences of underinvestment in health prevention when it comes to conditions like diabetes and obesity.

A recent Forbes article has alarmingly revealed that over 11% of the U.S. population is known to be diabetic. This translates to a staggering 37.3M people.
There’s also 8.5M people that have diabetes but have yet to be diagnosed. Diabetes has been on the rise for a while because of both the aging of the U.S. population -- as well as expanding waistlines.
This is a dire and costly problem. People who are diagnosed with diabetes incur average medical expenditures of $16,752 per year of which $9,601 are attributed to the disease!
The CDC has also found that an astronomical 96M adults in the U.S. are PRE-diabetic and 80% of these people have no idea that they have it.
It’s going to take some serious problem solving to solve this crisis but emerging med-tech company Nemaura Medical, Inc. (NASDAQ: NMRD) has a solution.
NMRD is addressing this huge problem with a non-invasive daily wear continuous glucose sensor.
The sheer convenience of the company’s wearable could be a game changer for millions of people. The device targets:
- A clinical market of over 60M people in Europe who have type 2 diabetes.
- A pre-diabetes and obesity market of over 90M people in the USA.

The USA market is currently the largest market opportunity for NMRD who could quickly turn into a household name in disease treatment and prevention with its wearable device.
With a non-invasive solution to help millions of people who suffer from chronic health conditions, Nemaura Medical Inc. (NASDAQ: NMRD) may be one of the brightest med tech stocks hiding on the NASDAQ!
More Reasons to Have NMRD on Your Radar Include:
- The company has submitted a PMA (Premarket Approval Application) for sugarBEAT® to the US FDA. proBEAT™ combines non-invasive glucose data processed using artificial intelligence under a digital healthcare subscription service as part of its BEAT® diabetes program.
- sugarBEAT® is non-invasive, affordable, and quite accommodating to a user’s daily routine, it is expected to have wider appeal to non-insulin using patients with diabetes, those with prediabetes, and a segment of insulin users who dislike competing CGMs (continuous glucose monitoring) owing to their invasiveness, cost, and inflexibility.
- The Company sits at the intersection of the global Type 2 diabetes market that is expected to reach nearly $59 billion by 2025, the $50+ billion pre-diabetic market, and the wearable health-tech sector for weight loss and wellness applications that is estimated to reach $60 billion by 2023. These are all massive markets.
- Obesity and diabetes are a burden on the U.S. healthcare economy but NMRD’s technology could help significantly lessen the burden. The company’s wearable device has the potential to help people reverse diabetes and manage weight.
- The successful use of feedback from glucose monitors for weight loss and type 2 diabetes reversal is well documented in both the clinical and non-clinical space and has been noted to increase user engagement.
- There is increasing demand globally from program providers to differentiate their programs with sensors that can provide feedback on overall health and wellness to potentially increase health outcomes and returns on investment for program providers.
- NMRD’s Platform uses precision microsystems-based technology that has taken decades to perfect in academia, and only recent advances in manufacturing have allowed these technologies to be cost-efficiently mass produced.
- Pilot studies integrating proBEAT glucose sensors as part of a metabolic health and wellbeing program are already currently running in collaboration with the UK’s National Health Service.
- The company has developed Miboko, (www.Miboko.com), a digital healthcare program with the glucose sensor at its heart. NMRD believes Miboko addresses a significant mass market opportunity that they believe could benefit roughly a third to half of the population by using a non-invasive glucose sensor to measure and monitor a user’s metabolic health score, which is based on glucose tolerance or insulin resistance.
- Miboki has a competitive landscape as a holistic Metabolic health approach to weight loss and better health. The weight-loss app Noom has over 50 million subscribers and over $400M in revenue!
- The company recently reported initial patient data this week from the UK NHS Miboko study that demonstrated weight loss in 100% of the participants!
There is substantial room for growth for Nemaura Medical Inc. (NASDAQ: NMRD) as it zooms in on the diabetes care market.
Notables in the diabetes care market include DexCom (DXCM) Medtronic (MDT) and Abbott Laboratories (ABT).
Halfway through 2022, DexCom reported revenue of $1.3 billion. Abbott said In the second quarter, sales of its FreeStyle Libre systems totaled $1.1 billion, up 18.7% year over year. Over the past 10 years, Medtronic has delivered a total return of 138%.

Company Overview
Nemaura Medical evolved from a microsystem-based drug delivery platform that was developed in 2011.
Since then, the company has expanded the platform to allow the monitoring of multiple chemicals in the blood, without using needles.
One of the great advantages of NMRD’s product, apart from eliminating the need to draw blood samples or inserting needles or sensors into the skin, is that a person can wear the device on whatever day they choose and for however long they choose.
This is a unique feature of the device that has vast potential to change the way people manage chronic disease conditions such as diabetes.
Those with prediabetes or obesity concerns, or even those looking to maintain better health through more careful glucose control, may benefit from the company’s Miboko, (www.Miboko.com) health program.
How one’s body metabolizes sugar is the main influencer of one’s appetite, weight, sleep quality, and energy and mood levels and even plays a critical factor in chronic diseases (beyond just diabetes), such as heart disease and dementia.
Advantages:
- Revenue generating and ramp-up in revenues anticipated through sales and potential new partnerships.
- First mover advantage with the non-invasive sensor platform.
- Simple subscription-based revenue generation models.
- Highly Scalable business model with potential for rapid growth and product introductions in the consumer space (non-regulated market) as well as in the clinical space.
Corporate Highlights
- Entered into a term sheet with Eversana to begin strategy development and subsequent launch of the BEATdiabetes program and the company’s unique daily wear proBEAT sensors in the U.S. The launch is expected in the first calendar quarter of 2023 and will target insurers and corporate clients. Eversana is a pioneer of next-generation commercial services to the global life sciences industry with its 6,500-person sales force.
- Received a provisional purchase order from TPMENA, the company’s Middle East/North Africa licensee for the company’s sugarBEAT® system, for 1.7 million sensors and 17,500 devices. The purchase order is contingent on the regulatory approval in the Kingdom of Saudi Arabia, which is expected in the coming months.
- Entered into an amendment of an existing $20 million note purchase agreement whereby the maturity date was extended from February 2023 to July 2024, increasing the company’s cash runway without requiring additional immediate capital raises.
BEAT® Technology Platform
Convenient, timely and painless, NMRD’s needle-free patented BEAT® sensor is applied to the skin via a small un-obtrusive patch and measures.
Transmitting this data to users and / or healthcare professionals via a Smartphone App, the technology will allow for medical conditions and chronic diseases to be monitored for better disease management or treatment.
Encouraging compliance due to its ease and flexibility of use, the company’s technology is expected to provide substantial savings to patients while potentially helping to significantly reduce future health complications from many medical conditions.
Applications of the technology include glucose monitoring, temperature monitoring, lactate monitoring, alcohol monitoring, and substance abuse monitoring.
Nemaura Medical aims to bring a multitude of products in the diagnostic field to global markets. This will be combined with world-class digital offerings so that we can play a significant role in the prevention and management of chronic disease conditions.
Company’s Technologies
Digital solutions for weight loss and potential reversal of Type 2 Diabetes and Pre-diabetes
A world-class program that was originally developed at the world-leading center for diabetes, the Joslin Institute.
This is a digital program that comes with over a decade of clinical evidence demonstrating excellent efficacy. The company has combined this program with its glucose-monitoring platform to bring an outstanding product to market to help people with diabetes manage their condition and potentially reverse Type 2 diabetes.
Glucose monitoring solutions for Type 2 Diabetes prevention and reversal
With over 420 million people worldwide with diabetes and the number of pre-diabetes cases at almost three times this number, diabetes is an urgent global health crisis.
Combining clinical research with patient-friendly technology, sugarBEAT® delivers a non-invasive, affordable and flexible method of blood glucose tracking for improved diabetes management.
Continuous Lactate Monitoring for Athletic Performance (Non-medical)
Lactic acid is a key performance indicator for the body and an indicator of how well muscles react to long-term exertion and recovery. Well-trained athletes and regular sports players are very efficient at faster lactate ‘recycling’ for extra energy (ATP). The company plans to launch its lactate sensor to the sports & personal training market.
Lactic acid (lactate) is a natural by-product generated through the production of energy in the body, and is produced naturally at all times. The relationship of lactic acid to the ability of the body to perform must be assessed in two parts: (1) the function of the lactate itself, and (2) the adverse effects of the hydrogen ion produced in the reaction that creates lactic acid.
Continuous Lactate Monitoring in Disease State (Medical)
An increase in blood lactate levels is also a marker of critical disease states. Recent publications have indicated the presence of elevated lactate levels in patients with COVID-19 infection.
Continuous lactate monitoring has applications in various disease states and critical care, including the monitoring of progression in viral infection, sepsis and septic shock, and COVID-19 infection.
The company has developed a lactate sensor that is being integrated into its BEAT® platform.
Continuous temperature monitoring for tracking viral disease progression and aiding fertility
A person’s body temperature says a lot about their health.
Several diseases, including COVID-19, are characterized by a fever (an increase in body temperature), meaning that temperature monitoring is a vital tool in the detection, diagnosis, and, consequently, in the prevention of the spread of disease.
Core body temperature measurements are used to track the course of a disease and allows medical teams to analyze the effectiveness of treatments and proactively adapt to improve outcomes.
Future Product Opportunities
ALCOHOL MONITORING
Support personal health goals and provide warnings prior to driving.
Provide physicians with individual’s drinking habits.
Prevention of progression-to-alcohol-related disease
DRUG MONITORING
Monitoring the impact of drugs and personalized treatment plan for patients.
Global therapeutic drug monitoring device market estimated to reach $3.37B by 2024.
UK NHS Miboko Study
According to a news release from January 24th, entitled, “Nemaura Medical Reports Initial Patient Data from UK NHS Miboko Study that Demonstrates Weight Loss in 100% of Participants,” NRMD has revealed stellar initial results from patient studies of its metabolic health program Miboko with the National Health Service (NHS) in the United Kingdom.
The initial pilot program recruited 30 individuals that were classified as obese according to their BMI. The first cohort of 10 patients have been enrolled in the program for over 12 weeks. Results from this cohort indicate that these individuals achieved an average weight loss of 3.7 pounds and an average BMI improvement of 0.6 after 10 weeks, with 100% of participants achieving some weight improvement.
This level of average weight loss exceeds some of the publicly reported results in studies of other weight loss programs. As the Miboko program is optimized, average weight loss is expected to increase. The program is designed to continue for at least one year, with new patients being enrolled each month.
The Miboko program is the VERY FIRST to integrate a daily-wear non-invasive glucose sensor with a lifestyle app, which includes recording of food and drink, educational content, and an analytics platform.
The wellness program provides each user a metabolic score based on diet, exercise, and glucose response to food intake, which is determined by the sensor. Users are given personalized recommendations to help them lose weight, potentially avoid chronic health issues and enjoy a better quality of life.
"These results are incredibly encouraging, as they validate the approach that we developed some time ago that integrates the use of a non-invasive glucose sensor with personalized, app-driven coaching and analytics. We look forward to continuing the study with the National Health Service and anticipate seeing increasingly robust findings as we continue to follow these users and initiate new users each month."
- Faz Chowdhury, Ph.D., CEO
Marketing Strategy
Recent feedback suggests there is a significant business case to offer the Metabolic health program (from Miboko), and the proBEAT sensor and auto generated progress report as plug-ins to existing diabetes management and metabolic health programs.
In the USA alone there are over 20003 such organizations that the company can target. This would further minimize the need for infrastructure and associated costs to NMRD.
Management
Dr. Faz Chowdhury - Chief Executive Officer
Dr. Chowdhury has served as CEO and Chairman of the Board of Nemaura Medical Inc. since 2013. He has over 20 years of experience in the Pharmaceutical and Medical devices industry, taking products from concept to commercial launch. He is sole inventor on more than 100 granted and pending patents across 15 technology platforms within the medical device and pharmaceutical sectors and has authored Textbook Chapters on Nano-biosciences for Wiley and Elsevier. Dr. Chowdhury is a regular speaker at international conferences in this field and serves on the Board of Medilink East Midlands, UK. He holds a Masters in Microsystems and Nanotechnology from Cranfield University, UK, and a Doctorate from the University of Oxford on nano-medicine and drug delivery.
Dr Arash Ghadar - Chief Operating Officer
Dr Arash Ghadar has over 20 years of experience in management and leadership including senior technical roles in medical devices, oil & gas and automation. His main area of expertise is in the healthcare sector, both in OEM and contract design and manufacturing settings, covering all aspects of new product life cycle from R&D to product design and commercial manufacture. Dr Ghadar is responsible for all aspects of operations at Nemaura, from design and development to volume production.
Dr. Ghadar holds a First Class BSc degree and a Master’s degree with Distinction in Electronics and Control Systems, respectively. He also has a Ph.D. in Electronic Biosensors from the University of Warwick (UK). He is a non-executive director on the board of Medilink Midlands (UK), the Life Sciences industry association with a vision to stimulate the growth of the life sciences sector, and also sits on the Innovation Board of LLEP (Leicestershire Enterprise Partnership).
Prior to joining Nemaura, Dr Ghadar spent a decade in executive positions, managing design and commercialisation disciplines as an autonomous entity within a contract design and manufacturing setting. He was responsible for management of the related day-to-day operations, business planning, legal affairs, finance, sales, and business development. In his role he also led and managed numerous technical projects for healthcare and industrial customers, covering product development lifecycle to volume manufacture.
David Scott - Director of Commercial Development and Licensing
David Scott is a trained chemist with over 35 years of experience in the Pharmaceutical Industry, spanning deal brokering, marketing, strategic planning, finance, business development, and acquisitions. He is a skilled negotiator who has closed a number of major deals for inward and outward licensing of pharmaceutical products, delivery systems, and technologies. He has also provided licensing training for a number of multinational pharma companies and training organizations and has published widely (he is the author of the best-selling report, “Scrip’s Practical Guide to Pharmaceutical Licensing”). David is also an accredited “Certified Licensing Professional”.
IN SUMMARY
Nemaura Medical, Inc. (NASDAQ: NMRD) is creating affordable diagnostic and digital tools that could benefit many markets but currently and most significantly, the diabetes and pre-diabetes markets.
In recent years, wearables have become a big part of peoples’ everyday lifestyles. The FitBit, Apple Watch, all of these devices are game-changers for the person on the go who w+nts to manage their health.
The global diabetes devices market is predicted to hit around US$ 48.8 billion by 2030 from valued at US$ 28.30 billion in 2021, growing at a CAGR of 6.2% over forecast period 2021 to 2030. The global diabetes devices market is growing at a rapid rate along with the rising prevalence of diabetes among the global population across the globe.
With roughly 1.4 billion people classified as overweight and some 500 million considered obese worldwide, the potential impact on companies in many industries -- from biotechnology to food -- is enormous. Even governments are likely to engage in the battle against fat because obesity-related illnesses such as diabetes are placing an increasingly heavy burden on public medical insurance programs, according to a Merrill Lynch report.
According to the World Health Organization, diabetes is a major cause of kidney failure, stroke, and blindness. The rising awareness regarding the diabetes and diabetes management among the population is significantly boosting the demand for the diabetes devices across the globe.
Nemaura Medical Inc. (NASDAQ: NMRD) may be one of the most exciting med-tech and healthcare related stocks going under the radar right now!
THIS IS A PAID ADVERTISEMENT
NO INVESTMENT ADVICE
This website is wholly owned by scd media llc (d/b/a “smallcapsdaily.com”). Our reports are advertorials and are for general information purposes only. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. This disclaimer is to be read and fully understood before using our services, joining our email list, as well as any social networking platforms we may use. Please note as well: Small Caps Daily and its employees are not Registered Investment Advisors, broker-dealers, or member(s) of any association for other research providers in any jurisdiction whatsoever. release of liability: through use of this website, viewing or using you agree to hold Small Caps Daily, its operators, owners, and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources that we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. Small Caps Daily encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the company profiled or is available from public sources and Small Caps Daily makes no representations, warranties, or guarantees as to the accuracy or completeness of the disclosure by the profiled company. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provided herein. Instead, Small Caps Daily strongly urges you to conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Small Caps Daily’s full disclosure is to be read and fully understood before using Small Caps Daily's website, or joining Small Caps Daily's email or text list. From time to time, Small Caps Daily will disseminate information about a company via website, email, sms, and other points of media. By viewing Small Caps Daily's website and/or reading Small Caps Daily's email or text newsletter you are agreeing to this ----> https://smallcapsdaily.com/disclaimer/. All potential percentage gains discussed in any communications are based on calculations from the low to the high of the day. We are engaged in the business of marketing and advertising companies for monetary compensation. In compliance with section 17(b) of the securities act we are disclosing that we have been compensated a fee pursuant to an agreement between scd media llc and sea path advisory. Small Caps Daily was hired for a period beginning in March 2020 and ending in April 2020 to publicly disseminate information about Nemaura Medical Inc. via website, email, and sms. We were paid five thousand usd via ACH. Subsequently, Small Caps Daily was hired for a period in January 2023 to publicly disseminate information about Nemaura Medical Inc. via website, email, and sms. We were paid five thousand usd via ACH. Subsequently, Small Caps Daily was hired for a period beginning in January 2023 and ending in February 2023 to publicly disseminate information about Nemaura Medical Inc. via website, email, and sms. We were paid five thousand usd via ACH. We are also disclosing that Tradigital Marketing Group has been compensated a fee pursuant to an agreement between Tradigital and Nemaura Medical Inc. Tradigital was hired for a period beginning in March 2020 and ending in April 2020 to publicly disseminate information about Nemaura Medical Inc., via website, email, and SMS. Tradigital was paid seventy thousand USD via ACH. Tradigital owns Ten thousand restricted common shares of Nemaura Medical Inc., which are eligible for sale on 09/16/2020. It is Tradigital’s intention to sell all of their shares once the restriction is lifted on 09/16/2020. Subsequently, Tradigital was hired for a period in January 2023 to publicly disseminate information about Nemaura Medical Inc., via website, email, and SMS. Tradigital was paid seventy-five thousand USD via ACH. Subsequently, Tradigital was hired for a period beginning in January 2023 and ending in February 2023 to publicly disseminate information about Nemaura Medical Inc., via website, email, and SMS. Tradigital was paid one hundred seventy-five thousand USD via ACH. Nemaura has the option to add an additional newsletter group on Thursday, February 9th, for a cost of twenty-five thousand USD. Read TraDigital’s disclosure in full HERE. Readers are advised to review sec periodic reports: forms 10-q, 10k, form 8-k, insider reports, forms 3, 4, 5 schedule 13d. Small Caps Daily is compliant with the can-spam act of 2003. Small Caps Daily does not offer investment advice or analysis, and Small Caps Daily further urges you to consult your own independent tax, business, financial, and investment advisors. investing in micro-cap, small-cap, and growth securities is highly speculative and carries an extremely high degree of risk. it is possible that an investor's investment may be lost or impaired due to the speculative nature of the companies profiled.the private securities litigation reform act of 1995 provides investors a safe harbor in regard to forward-looking statements. any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events, or performance are not statements of historical fact may be forward-looking statements. forward-looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. forward-looking statements in this action may be identified through the use of words such as projects, foresee, expects, will, anticipates, estimates, believes, understands, or that by statements indicating certain actions & quotes; may, could, or might occur. understand there is no guarantee past performance will be indicative of future results in preparing this publication, Small Caps Daily has relied upon information supplied by its clients, as well as its clients’ publicly available information and press releases which it believes to be reliable; however, such reliability can not be guaranteed. investors should not rely on the information contained on this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. the advertisements in this website are believed to be reliable, however, Small Caps Daily and its owners, affiliates, subsidiaries, officers, directors, representatives, and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of material facts from such advertisement. Small Caps Daily is not responsible for any claims made by the companies advertised herein, nor is Small Caps Daily responsible for any other promotional firm, its program, or its structure. Small Caps Daily is not affiliated with any exchange, electronic quotation system, the securities exchange commission, or finra.