With an innovative approach to defeating viruses, including COVID-19, this company may soon become a DOMINANT leader in nanomedicine and antiviral therapy!
The transmission of viruses has been increasing and the next pandemic could be much sooner and more severe than we think.
This puts the spotlight on emerging NYSE company NanoViricides, Inc (NYSE: NNVC) who is DISRUPTING the antiviral market and targeting several others with their nanoviricide® technology.
This technology may change the way humans fight against viruses forever!

With over 15+ years of experience in nanomedicine and antiviral therapy, NanoViricides, Inc. (NYSE: NNVC) is a global leader in the development of nanomedicine drugs against viruses.
The company’s platform technology defines a novel mechanism enabling first-in class drugs against viruses!
The potential of this nanoviricide® technology means that reliance on vaccines and our own immune system could become a thing of the past as the company will offer a more powerful means to override and eradicate viruses already present in the host!
One reason to have the company high on your radar is because current developments may bring NanoViricides, Inc. (NYSE: NNVC) discoveries and anti-COVID treatments straight to the market SOON!
It was in September 2021 that NanoViricides, Inc. announced it has completed the process of licensing the human Coronavirus field for drug development and commercialization from TheraCour Pharma, Inc.
Since then, the company has been rapidly moving toward human clinical trials with their two drug candidates: NV-CoV-2 N and NV-CoV-2-R, with excellent results in animal trials promising cures in humans!
NV-CoV-2 and NV-CoV-2-R were found to be highly effective against a totally lethal coronavirus lung infection in an animal model study in rats based on multiple indicators.
Treatment with the standard remdesivir formulation (Gilead Veklury® ) extended lifespan by only 2 days, while treatment with NV-CoV-2 and NV-CoV-2-R extended the lifespan by 8.5 and 10.5 days respectively!
That is a 400% to 500% more effectiveness than remdesivir, so strong that it should result in a cure in humans!
Read more about Nanoviricides' Rapid Movements Towards Clinical Stages
With NanoViricides having an active focus on developing treatments for SARS-CoV-2 patients, the company has already successfully developed safe oral gummies with NV-CoV-2 N that are to be tested in human clinical trials.

NOW IS A CRITICAL TIEM TO BE PAYING ATTENTION!
Led by NanoViricides inventor and President, Dr. Anil R. Diwan, and his heavily experienced management team, NanoViricides, Inc. (NYSE: NNVC) is well-positioned to defeat COVID-19 among other viruses.
NanoViricides, Inc.’s quick action against the COVID-19 could alleviate the requirements for semi-annual vaccines and overpower the virus’ persistence due to mutations!
WHAT DOES THIS MEAN?
This means that we can see WIDE acceptance and HIGH demand amongst infected patients for these drug products. With the highly transmissible coronavirus variants, almost 30-50% of the population will be infected, whether vaccinated or not.
On top of that - NanoViricides, Inc’s developing product line could help fight against stronger and upcoming mutations of SARS-CoV-2 - alleviating individuals from the stress of long quarantines and overpowering waves of re-infection.
THE COMPANY DOESN’T STOP AT COVID
Present in the antiviral therapy space for over 15+ years, the company’s testing and research has targeted multiple viral markets, including HIV, Shingles, and more.
As you see, NanoViricides, Inc.’s’ diverse and promising product pipeline is actively pursuing a multitude of infectious viruses through a variety of different and accessible inceptions.
THE TOP REASONS TO WATCH THIS COMPANY CLOSELY:
- Once NanoViricides' drug products hit the market - it could change the world!
- Over 15+ years of experience in nanomedicine and antiviral therapy developments.
- Current fast moving drug candidate developments and indications for treatment of SARS-CoV-2 patients.
- Possession of a strong, diverse, and promising product pipeline that addresses the world’s most infamous and infectious viruses.
- Antiviral therapy and nanomedicine market is expected to experience exponential growth by 2026!
- The company is one of a few biopharma companies that has its own cGMP-compliant manufacturing facility. The Company intends to produce its drugs for clinical trials in this facility. The Company has the capability to produce sufficient drugs for about 1,000-5,000 patients in a single batch of production, depending upon the drug and the dosage!
- NanoViricides, Inc.’s developments are sustainable beyond the COVID-19 as its diverse product pipeline targets several markets simultaneously:
- The global HIV drugs market is projected to grow from $30.46 billion in 2021 to $45.58 billion in 2028 at a CAGR of 5.9% in forecast period, 2021-2028.
- The global influenza medication market size stood at USD 889.2 million in 2018 and is projected to reach USD 993.7 million by 2026, exhibiting a CAGR of 2.2% during the forecast period.
- The shingles treatment market is expected to witness a high growth over the forecast period owing to the increasing prevalence of shingles. The US spent over $2.5 billion on treatment of shingles patients in 2016. The shingles drug market is estimated at $1 billion in 2017 increasing at the rate of 2%p..a. in the USA alone.
- COVID-19 has resulted in “long COVID”, a large swath of the world population with immune compromise. Shingles rates are rising because of this.
- The Company reported an increase of cash and cash equivalent current assets balance to approximately $17 Million, and additional assets of $8.93 Million in Property and Equipment (P&E) assets. Total Current liabilities were $0.344 Million
- Strong P&E assets comprise their cGMP-capable manufacturing and R&D facility, listed at $9 Million on the books. To replace it would cost more than $30 Million today!
- Led by inventor of nanoviricides technology: President, Dr. Anil Diwan and managed by a highly effective team with decades of entrepreneurial, pharmaceutical, and nanomedicine experience.
PLUS…. NNVC Has Sufficient Cash, and NV-CoV-2 Coronavirus Drug Candidate Clinical Trials Application is Ready!
The Company reported recently that it had approximately $17.5 Million of current assets (cash, cash equivalents, and prepaid expenses) and current cash liabilities of approximately $0.34 million, as of December 31, 2022. Also as of that date, the Company had no debt and stockholder's equity was approximately $26.46 million.
NO DEBT AND CASH ON HAND TO MOVE FORWARD….
Keep on reading to see why NanoViricides, Inc. (NYSE: NNVC) may be one of the most exciting undiscovered small-cap companies in the market today!
Greetings Investors,
The antiviral therapy market is on the verge of a transformative disruption.
Within the next decade, rapid and exponential developments in the intersection of antiviral drugs and nanomedicine will revolutionize the way in which positive patients are treated and protected from COVID-19 and other various viral infections.
As the pandemic has placed the world in a state of vulnerability and uncertainty, Big Pharma such as Moderna, Pfizer, and Johnson & Johnson offered their solutions with vaccines.
However, with the need to receive boosters, still wear masks, and quarantine, vaccines only serve as one of the protective measures rather than one which assures that you don’t get infected with the virus. In fact, with the variants emerging, the vaccines only promise to reduce the possibility that you will be hospitalized!
And the variants have defeated most of the available drugs already. The antibody drugs have become largely or wholly ineffective. Other drugs have limited effect. Worse, they newer drugs come with warnings that they cannot be used in many patient populations.
With greater demand and the need for cutting-edge solutions against COVID-19 and additional viruses, NanoViricides, Inc’s abundant experience and quick developments is even more reason to keep an eye on this company.
NanoViricides’ foundation is built upon intellectual property, licensed patents, and expertise that positions the company as a strong, reliable, and innovative drug developer in the antiviral and nanomedicine space.
Their most recent development of oral gummies to combat SAR-CoV-2 in positive patients is one of many leading-edge developments that NanoViricides plans to bring to the market!
Nanoviricides, Inc.’s patented and cutting-edge technology positions this company to be a dominant leader in antiviral therapy and nanomedicine so add the company to your radar!

ANTIBODIES AND VACCINES ARE OUTDATED
Antibodies have been developed as drugs against viruses. However, each antibody only binds by two points to the virus, and destruction of the complex requires effective immune function, which is not the case in sickness.
Vaccines only train the body into producing antibodies against the virus in the vaccine. Antibodies and vaccines are easily overcome by viruses by mutating in the field, hence the need for annual influenza vaccine updates. And, worse, the need for repeated boosters with coronavirus vaccines.
While Moderna and Pfizer have dominated coronavirus field with their development of the COVID-19 vaccines, NanoViricides, Inc. is taking a more innovative approach beyond antibodies and vaccines.
See Below for Nanoviricides' Unique, Novel, Post-Immunotherapeutic “Bind-Encapsulate-Destroy” Mechanism
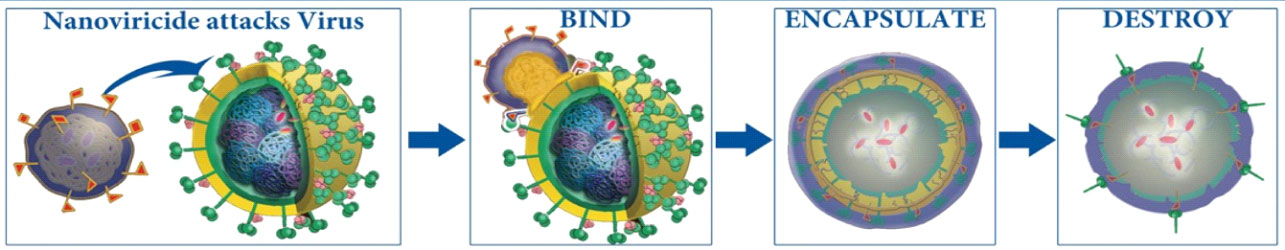
MEET BIOTECH’S NEW HERO: NANOVIRICIDES, INC.
NanoViricides, Inc. is a development stage company with a unique nanomedicine technology. The company is developing nanotechnology-based biomimetic anti-viral medicines, called “nanoviricides.”
In contrast to immunizations, A nanoviricide is a Nanomachine that completes the task of destroying a virus WITHOUT the help of the patient’s immune system.
This solves the key issues of drug resistance from viral mutations as nanoviricides drugs are empowered by biomimetic technology.
Designed by NanoViricides to “fool” a virus, the nanoviricides attach to the agent in the same way that the virus normally attaches to receptors on a cell surface.
The nanoviricide dismantles and eliminates the virus’ effect on the host by directly attacking the virus at multiple points.

MAJOR AND TIMELY DEVELOPMENTS AMIDST COVID-19
In response to the COVID-19 pandemic, NanoViricides, Inc .is applying their expertise and technology to currently focus and advance their development to treat SARS-CoV-2.
In late March 2021, the company developed two COVID-19 antiviral drugs with promising results in animal trials. Since then the Company is diligently developing two drug candidates against SARS-CoV-2, NV-CoV-2 and NV-CoV-2-R. Both are ready for clinical trials now. NV-CoV-2 will be the first in clinical trials very soon.
With sufficient cash and no debt, NanoViricides is ACTIVELY utilizing their strong financial position to advance their drug candidates to human clinical trials for treatment of patients with SARS-CoV-2.
Prioritizing unmet medical needs, NanoViricides has successfully developed "oral gummies” with NV-CoV-2 -- a broad-spectrum, pan-coronavirus drug candidate.
To aid immuno-compromised groups, such as children and older patients, NanoViricides' oral gummies provide an accessible and acceptable means to fight SARS-CoV-2 in contrast to pills or vaccines.
In addition, NanoViricides has also developed NV-CoV-2 formulations for injection, infusion, and direct lung inhalation using a simple mouthpiece, designed to benefit severely ill and hospitalized patients
NANOVIRICIDES, INC. PROMISING PRODUCT PIPELINE
NanoViricides, Inc. is diligently developing AN ACCESSIBLE, SAFE, and DIVERSE catalog of products.
With NanoViricides, Inc. broad pipeline, the company is enabled by the proprietary and unique post-immunotherapeutic "bind-encapsulate-destroy" technology platform, well-positioning the Company to be an overall therapeutic leader across the anti-viral ecosystem. (click here to read more)
NanoViricides, Inc. has already demonstrated its leadership within the antiviral therapeutic space by developing a diverse and promising product pipeline that attacks viral infections from ALL ANGLES.
From Herpes and Ebola to Shingles, HIV, and severe influenzas, NanoViricides, Inc. currently has multiple drug candidates in development to treat various viruses through various means such as topical creams, eye drops, injections, and gummies.
NanoViricides’ promising developments of these diverse products are projected to meet the individual and personalized needs of patients through these EFFECTIVE and ACCESSIBLE treatments.


KEY ADVANTAGES OF NANOVIRICIDES, INC.
With market growth in both antiviral therapy and nanomedicine, NanoViricides' current developments have given the company SEVERAL advantages for success.
EXPERTISE: Founded in 2005, NanoViricides, Inc. has been working for more than a decade to develop antiviral treatments that are like nothing on the market today.
SPEED TO THE MARKET: Stepping closer towards human clinical trials to test developing treatments for SARS-CoV-2, NanoViricides, Inc. could see its “nanoviricides” reach the market relatively quickly amidst the pandemic.
POTENTIAL FUNDING: The COVID-19 pandemic has led to a flood of funds, provided by both public and private agencies, in an attempt to end the pandemic. As a result, NanoViricides, Inc. stands as the beneficiary of one or more financial programs that we are seeing hit the tape.
FUTURE REVENUE: With plans to bring their nanoviricides to the market, NanoViricides, Inc. will generate SIGNIFICANT REVENUE due to current demand in antiviral therapy and nanomedicine.
As their diverse product line offers patients both accessibility and acceptability, NanoViricides' products will be in high demand once publicly available.
DIVERSE APPROACH: NanoViricides, Inc. taps into several incredible valuable markets: COVID-19, HIV, Influenza, Dengue, and Shingles.
Through their diverse approach to produce nanoviricides to attack a wide range of infectious viruses, Nanoviricides, Inc. increase their likelihood of success once their products hit the market.
All it takes is for NanoViricides to release one of their developments in just ONE of these areas!!
SUSTAINABILITY BEYOND COVID: NanoViricides Inc.’s “nanoviricides” are expected to be effective in a wide range of indications such as their HerpeCide™, HIVCide™ and FluCide™ franchises.
Their indications include BOTH injectable and ORAL delivery drugs that are expected to be effective REGARDLESS of mutations.
TAPPING INTO A MASSIVE MARKET
NanoViricides, Inc.’s promising pipeline is targeting a multi-billion antiviral therapy market.
With the antiviral area currently in the spotlight due to the search for effective COVID-19 treatments , the global anti therapy market is valued for $48.1 million in 2018 and is estimated to garner $79.8 million by 2026, rising at a CAGR of 6.7% from 2019 to 2026.
The extensive growth of the antiviral therapy market is mainly attributed to the increasing prevalence of human immunodeficiency viruses (HIV) infections and hepatitis C, which NanoViricides is actively pursuing in addition to COVID-19.
NanoViricides, Inc. current programs for HIV, Dengue, Ebola/ Marburg and other viruses comprise a market size of $40~70 Billion.
Additionally, NanoViricides HerpeCide™ Program has its first drug candidate for treatment of shingles, NV-HHV-101, with a topical cream.
The market size for this program is more than ~$10Bn, with the market size for NV-HHV-101 estimated at between $0.5Bn~$2Bn by independent market studies.
NANOMEDICINE: FUTURE OF HEALTHCARE
As COVID-19 continues to spread across the globe, increased demand for nanomedicine has led to the strong growth of the global nanomedicine market.
Looking forward, the market is projected to grow at a CAGR of 10% during 2021-2026.
Extensive research, contributed by NanoViricides, Inc., and development (R&D) activities in the field of biotechnology have anticipated to drive the market toward growth.
Research shows that nanomedicine is HIGHLY beneficial in the treatment of immunological and infectious disease -- NanoViricides Inc.’s expertise!
WHY NANOVIRICIDES, INC. WILL TRANSFORM THIS MARKET
NanoViricides, Inc. programs address large markets within the antiviral space that could result in treatment-transformations and market size explosions.
Using the nanoviricides® platform technology, NanoViricides, Inc. has developed drug candidates that target a fruitful and expansive market as the company attacks the world’s most pervasive viruses.
With anticipated market explosion on the horizon, NanoViricide’s anti-Herpes drugs franchise, FluCide franchise, and other pipeline products will reap the benefits of this market boom.
Setting its sights on expanding its current portfolio with COVID-19 drug candidates, NanoViricides has already established itself as a transformative leader in the antiviral market.

A FOUNDATION BUILT UPON LICENSING AND INTELLECTUAL PROPERTY
NanoViricides, Inc. 's drug development business model was formed in May 2005 with a license to the patents and intellectual property held by TheraCour Pharma, Inc. that enabled creation of drugs engineered specifically to combat viral diseases in humans.
This exclusive license from TheraCour serves as a foundation for NanoViricides’ intellectual property.
NanoViricides, Inc. possesses WORLDWIDE exclusive license to this technology for several drugs with specific targeting mechanisms for the treatment of several human viral diseases including VZV (shingles), HSV-1 and HSV-2, as wellas Coronaviruses.
Nanoviricides intends to perform the regulatory filings and own all the regulatory licenses for the drug candidates it is currently developing. The company will develop these drugs in part via subcontracts to TheraCour, the exclusive source for these nanomaterials. (Read PR Here)
CGMP-COMPLIANT MANUFACTURING FACILITY
NanoViricides, Inc. is one of a few biopharma companies that has its own cGMP-compliant manufacturing facility.
It’s’ nanomedicine manufacturing capabilities largely determined its product pipeline, which focuses on drug candidates that have shorter development timelines and could be easily manufactured in-house.
The NanoViricides, Inc. capability to produce sufficient drugs for about 1,000 patients in a single batch of production, depending upon dosage.
This production capacity is anticipated to be sufficient for first-in-human use in the current SARS-CoV-2 pandemic, as well as for the anticipated clinical trials of NV-HHV-101.
The facility’s R&D capabilities include screening of numerous molecules that could serve as virus-binding ligands, and NanoViricides Inc. has amassed a large library of potential candidates.
This library, as well as a state-certified biosafety level (BSL)-2 lab with three separate virology suites, enables work on three different viruses at once.
“We are able to go from the lab bench to cGMP manufacturing in a matter of three-to-six months,” Dr. Diwan contends, noting that while NanoViricides’ initial manufacturing capacity was 500 grams a year, it’s now capable of manufacturing several 25 kg batches per year.

MEET CREATOR AND PRESIDENT OF NANOVIRICIDES, INC.
Creator and developer of nanomedicine technologies, Dr. Anil R. Diwan co-founded NanoViricides and currently serves as the company’s President and Executive Chairman.
Diwan has over 45+ years of experience in entrepreneurship and bio-pharmaceutical R&D. Beginning with the invention of novel polymeric micelle-based nanomedicine technologies in 1991, it wasn’t until 2005 that NanoViricides, Inc. was born.
Since Dr. Diwan’s efforts for the company’s 2013 up listing from the OTC Markets to NYSE-American, Dr. Diwan has led several of the financing efforts since 2010 and has raised over 100+ MM in equity financing.
Under Dr. Diwan’s leadership, Nanoviricides, Inc. has been able to keep both administrative and R&D costs at extremely low levels, resulting in expanding the drug pipeline every day.
Prior to co-founding NanoViricides, Inc, he founded TheraCour Pharm, Inc. - a privately held company focused in nanomedicines and cell-targeted drug delivery, and AllExcel, Inc., a company with diverse portfolios including nanomedicines and device technologies.
He has won several NIH SBIR (Small business innovation research) grant awards and holds a Ph.D. from Rice University, Tx, a B.Tech. from Indian Institute of Technology, Mumbai (IIT-B), India, and has consistently held high scholastic ranks and honors.
He has over 60 patents issued internationally resulting from three fundamental international patent applications.
- Dr. Anil R. Diwan Leads NanoViricides in Fight Against COVID-19
- NanoViricides’ President Dr. Anil Diwan was Interviewed by Proactive Investors About the Company’s Antiviral Drug Development Against SARS-CoV-2 to Treat COVID-19
- NanoViricides’ President Dr. Anil Diwan was Interviewed by Proactive Investors About the Company’s Antiviral Drug Development Against SARS-CoV-2 to Treat COVID-19

BACKED BY A HIGHLY EFFECTIVE MANAGEMENT
Meet the rest of Nanoviricides’ expert-driven management team!
Interlaced with decades of entrepreneurship, nanomedicine, and pharmaceutical experience, this team has led Nanoviricides to make innovative and groundbreaking developments in antiviral therapy since its genesis.
Meeta R. Vyas, SB, MBA, CFO
- Former CEO of Signature Brands, a public company she turned around, was acquired by Sunbeam Corp
- ex GM of GE Appliances Range products with sales over $1 Billion; doubled operating income
- ex-Principal with Gores Operations Group, a PE firm based in Los Angeles.
Randall W. Barton, PhD, Chief Scientific Officer
- Ex-Director of In-Vivo Cardiovascular Research at Boehringer-Ingelheim
- An expert in receptor-based drug design
- More than 60 scientific publications, 5 patents and 3 patent applications.
Jayant Tatake, PhD, Vice President of R&D
- Co-inventor of the Company’s nanomedicine technologies.
- Over 23 years of experience with production of pharmaceuticals from lab scale through cGMP manufacture
- Experience running cGLP QA/QC Labs at a Pharma Company and at a CRO. PhD from UICT-Bombay
THE BOTTOM LINE
NanoViricides Inc (U.S. - NNVC) is set to be a disruptor within the antiviral therapy and nanomedicine space and is positioned to capture a $80B market once products are publicly available.
With its diverse and promising product pipeline and timely drug developments for SARs-CoV-2, NanoViricides' future drug products could change the way we combat viruses and upcoming mutations.
Advancements of nanomedicine are around the corner as we know it and Nanoviricides' 15+ years of development and invention of nanoviricides puts the company ahead of the game.
As we have seen, NanoViricides, Inc. (NYSE: NNVC) are quickly developing drug products with their unique and powerful technology and preparing to meet demands for long-term solutions to COVID-19.
Success have already been achieved in animal trials and NanoViricides, Inc. is not hesitating to move forward.
Their rapid advancement towards human clinical trials is something you do not want to miss!
Their in-house manufacturing capabilities. strong financial position, and ownership of intellectual property only contributes to NanoViricides, Inc. diligent responsibility to current and future health situations as their product pipeline continues to expand with cutting-edge solutions to combat viral infections of the past, present, and future.
NanoViricides, Inc. (NYSE: NNVC) is on its way of becoming a leading innovator in the world of nanomedicine and antiviral therapy as its developments and technology could revolutionize the way in which we combat and eradicate viruses today!
This website is wholly owned by tradigital marketing group, inc. (d/b/a “tradigital ir”). Our reports are advertorials and are for general information purposes only. never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. The disclaimer is to be read and fully understood before using our services, joining our email list, as well as any social networking platforms we may use. please note well: tradigital ir and its employees are not registered investment advisors, broker-dealers, or member(s) of any association for other research providers in any jurisdiction whatsoever. release of liability: through use of this website, viewing or using you agree to hold tradigital ir, its operators, owners, and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources that we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. tradigital ir encourages readers and investors to supplement the information in these reports with independent research and other professional advice. all information on featured companies is provided by the companies profiled or is available from public sources and tradigital ir makes no representations, warranties, or guarantees as to the accuracy or completeness of the disclosure by the profiled companies. none of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provided herein. instead, tradigital ir strongly urges you to conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. tradigital ir’s full disclosure is to be read and fully understood before using tradigital ir's website, or joining tradigital ir's email or text list. From time to time, tradigital ir will disseminate information about a company via website, email, sms, and other points of media. By viewing tradigital ir's website and/or reading tradigital ir's email or text newsletter you are agreeing ----> https://tradigitalir.com/disclaimer-tmg/. all potential percentage gains discussed in any communications are based on calculations from the low to the high of the day. We are engaged in the business of marketing and advertising companies for monetary compensation. in compliance with section 17(b) of the securities act we are disclosing that we have been compensated a fee pursuant to an agreement between tradigital and nanoviricides, inc. tradigital was hired for a period beginning august 2019 and ending november 2019 to publicly disseminate information about nanoviricides, inc., via website, email, and sms. We were paid five thousand usd each month, via ach. We owned sixty thousand restricted common shares of nanoviricides, inc., which were eligible for sale on 02/13/2020. We sold this entire position as of 3/4/2022. tradigital was hired for a period beginning january 2020 and ending january 2021 to publicly disseminate information about nanoviricides, inc., via website, email, and sms. We were paid five thousand usd each month, via ach. We owned fifty thousand restricted common shares of nanoviricides, inc., which were eligible for sale on 06/02/2020. We sold this entire position as of 3/4/2022. tradigital was hired to publicly disseminate information about nanoviricides, inc., via website, email, and sms. We were paid twenty-five thousand usd, via ach in february 2022 and the marketing will last for thirty days. We do not own any shares of nanoviricides, inc. Readers are advised to review sec periodic reports: forms 10-q, 10k, form 8-k, insider reports, forms 3, 4, 5 schedule 13d. tradigital ir is compliant with the can-spam act of 2003. tradigital ir does not offer investment advice or analysis, and tradigital ir further urges you to consult your own independent tax, business, financial, and investment advisors. investing in micro-cap, small-cap, and growth securities is highly speculative and carries an extremely high degree of risk. it is possible that an investor's investment may be lost or impaired due to the speculative nature of the companies profiled.the private securities litigation reform act of 1995 provides investors a safe harbor in regard to forward-looking statements. any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events, or performance are not statements of historical fact may be forward-looking statements. forward-looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. forward-looking statements in this action may be identified through the use of words such as projects, foresee, expects, will, anticipates, estimates, believes, understands, or that by statements indicating certain actions & quotes; may, could, or might occur. understand there is no guarantee past performance will be indicative of future results in preparing this publication, tradigital ir has relied upon information supplied by its clients, as well as its clients’ publicly available information and press releases which it believes to be reliable; however, such reliability can not be guaranteed. investors should not rely on the information contained on this website. rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, tradigital ir and its owners, affiliates, subsidiaries, officers, directors, representatives, and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of materials facts from such advertisement. tradigital ir is not responsible for any claims made by the companies advertised herein, nor is tradigital ir responsible for any other promotional firm, its program, or its structure. tradigital ir is not affiliated with any exchange, electronic quotation system, the securities exchange commission, or finra.
THIS WEBSITE IS WHOLLY OWNED BY TRADIGITAL MARKETING GROUP, INC. (D/B/A “TRADIGITAL IR”). OUR REPORTS ARE ADVERTORIALS AND ARE FOR GENERAL INFORMATION PURPOSES ONLY. NEVER INVEST IN ANY STOCK FEATURED ON OUR SITE OR EMAILS UNLESS YOU CAN AFFORD TO LOSE YOUR ENTIRE INVESTMENT. THE DISCLAIMER IS TO BE READ AND FULLY UNDERSTOOD BEFORE USING OUR SERVICES, JOINING OUR EMAIL LIST, AS WELL AS ANY SOCIAL NETWORKING PLATFORMS WE MAY USE. PLEASE NOTE WELL: TRADIGITAL IR AND ITS EMPLOYEES ARE NOT REGISTERED INVESTMENT ADVISORS, BROKER-DEALERS, OR MEMBER(S) OF ANY ASSOCIATION FOR OTHER RESEARCH PROVIDERS IN ANY JURISDICTION WHATSOEVER. RELEASE OF LIABILITY: THROUGH USE OF THIS WEBSITE, VIEWING OR USING YOU AGREE TO HOLD TRADIGITAL IR, ITS OPERATORS, OWNERS, AND EMPLOYEES HARMLESS AND TO COMPLETELY RELEASE THEM FROM ANY AND ALL LIABILITY DUE TO ANY AND ALL LOSS (MONETARY OR OTHERWISE), DAMAGE (MONETARY OR OTHERWISE), OR INJURY (MONETARY OR OTHERWISE) THAT YOU MAY INCUR. THE INFORMATION CONTAINED HEREIN IS BASED ON SOURCES THAT WE BELIEVE TO BE RELIABLE BUT IS NOT GUARANTEED BY US AS BEING ACCURATE AND DOES NOT PURPORT TO BE A COMPLETE STATEMENT OR SUMMARY OF THE AVAILABLE DATA. TRADIGITAL IR ENCOURAGES READERS AND INVESTORS TO SUPPLEMENT THE INFORMATION IN THESE REPORTS WITH INDEPENDENT RESEARCH AND OTHER PROFESSIONAL ADVICE. ALL INFORMATION ON FEATURED COMPANIES IS PROVIDED BY THE COMPANIES PROFILED OR IS AVAILABLE FROM PUBLIC SOURCES AND TRADIGITAL IR MAKES NO REPRESENTATIONS, WARRANTIES, OR GUARANTEES AS TO THE ACCURACY OR COMPLETENESS OF THE DISCLOSURE BY THE PROFILED COMPANIES. NONE OF THE MATERIALS OR ADVERTISEMENTS HEREIN CONSTITUTE OFFERS OR SOLICITATIONS TO PURCHASE OR SELL SECURITIES OF THE COMPANIES PROFILED HEREIN AND ANY DECISION TO INVEST IN ANY SUCH COMPANY OR OTHER FINANCIAL DECISIONS SHOULD NOT BE MADE BASED UPON THE INFORMATION PROVIDED HEREIN. INSTEAD, TRADIGITAL IR STRONGLY URGES YOU TO CONDUCT A COMPLETE AND INDEPENDENT INVESTIGATION OF THE RESPECTIVE COMPANIES AND CONSIDERATION OF ALL PERTINENT RISKS. TRADIGITAL IR’S FULL DISCLOSURE IS TO BE READ AND FULLY UNDERSTOOD BEFORE USING TRADIGITAL IR'S WEBSITE, OR JOINING TRADIGITAL IR'S EMAIL OR TEXT LIST. FROM TIME TO TIME, TRADIGITAL IR WILL DISSEMINATE INFORMATION ABOUT A COMPANY VIA WEBSITE, EMAIL, SMS, AND OTHER POINTS OF MEDIA. BY VIEWING TRADIGITAL IR'S WEBSITE AND/OR READING TRADIGITAL IR'S EMAIL OR TEXT NEWSLETTER YOU ARE AGREEING ----> HTTPS://TRADIGITALIR.COM/DISCLAIMER-TMG/. ALL POTENTIAL PERCENTAGE GAINS DISCUSSED IN ANY COMMUNICATIONS ARE BASED ON CALCULATIONS FROM THE LOW TO THE HIGH OF THE DAY. WE ARE ENGAGED IN THE BUSINESS OF MARKETING AND ADVERTISING COMPANIES FOR MONETARY COMPENSATION. IN COMPLIANCE WITH SECTION 17(B) OF THE SECURITIES ACT WE ARE DISCLOSING THAT WE HAVE BEEN COMPENSATED A FEE PURSUANT TO AN AGREEMENT BETWEEN TRADIGITAL AND PASITHEA THERAPEUTICS CORP. TRADIGITAL WAS HIRED FOR A PERIOD BEGINNING JANUARY 2022 AND ENDING MARCH 2022 TO PUBLICLY DISSEMINATE INFORMATION ABOUT PASITHEA THERAPEUTICS CORP. VIA WEBSITE, EMAIL, AND SMS. WE WERE PAID FIVE HUNDRED FORTY-FOUR THOUSAND USD VIA ACH. WE OWN ONE HUNDRED FIFTY THOUSAND RESTRICTED COMMON SHARES OF PASITHEA THERAPEUTICS CORP., WHICH ARE ELIGIBLE FOR SALE ON 03/18/2022. FOR THE PURPOSE OF THIS DISCLAIMER, WE SUGGEST THAT YOU ASSUME WE WILL SELL ALL OF OUR SHARES ONCE THE RESTRICTION IS LIFTED ON 03/18/2022. READERS ARE ADVISED TO REVIEW SEC PERIODIC REPORTS: FORMS 10-Q, 10K, FORM 8-K, INSIDER REPORTS, FORMS 3, 4, 5 SCHEDULE 13D. TRADIGITAL IR IS COMPLIANT WITH THE CAN-SPAM ACT OF 2003. TRADIGITAL IR DOES NOT OFFER INVESTMENT ADVICE OR ANALYSIS, AND TRADIGITAL IR FURTHER URGES YOU TO CONSULT YOUR OWN INDEPENDENT TAX, BUSINESS, FINANCIAL, AND INVESTMENT ADVISORS. INVESTING IN MICRO-CAP, SMALL-CAP, AND GROWTH SECURITIES IS HIGHLY SPECULATIVE AND CARRIES AN EXTREMELY HIGH DEGREE OF RISK. IT IS POSSIBLE THAT AN INVESTORS INVESTMENT MAY BE LOST OR IMPAIRED DUE TO THE SPECULATIVE NATURE OF THE COMPANIES PROFILED.THE PRIVATE SECURITIES LITIGATION REFORM ACT OF 1995 PROVIDES INVESTORS A SAFE HARBOR IN REGARD TO FORWARD-LOOKING STATEMENTS. ANY STATEMENTS THAT EXPRESS OR INVOLVE DISCUSSIONS WITH RESPECT TO PREDICTIONS, EXPECTATIONS, BELIEFS, PLANS, PROJECTIONS, OBJECTIVES, GOALS, ASSUMPTIONS OR FUTURE EVENTS, OR PERFORMANCE ARE NOT STATEMENTS OF HISTORICAL FACT MAY BE FORWARD-LOOKING STATEMENTS. FORWARD-LOOKING STATEMENTS ARE BASED ON EXPECTATIONS, ESTIMATES, AND PROJECTIONS AT THE TIME THE STATEMENTS ARE MADE THAT INVOLVE A NUMBER OF RISKS AND UNCERTAINTIES WHICH COULD CAUSE ACTUAL RESULTS OR EVENTS TO DIFFER MATERIALLY FROM THOSE PRESENTLY ANTICIPATED. FORWARD-LOOKING STATEMENTS IN THIS ACTION MAY BE IDENTIFIED THROUGH THE USE OF WORDS SUCH AS PROJECTS, FORESEE, EXPECTS, WILL, ANTICIPATES, ESTIMATES, BELIEVES, UNDERSTANDS, OR THAT BY STATEMENTS INDICATING CERTAIN ACTIONS & QUOTES; MAY, COULD, OR MIGHT OCCUR. UNDERSTAND THERE IS NO GUARANTEE PAST PERFORMANCE WILL BE INDICATIVE OF FUTURE RESULTS IN PREPARING THIS PUBLICATION, TRADIGITAL IR HAS RELIED UPON INFORMATION SUPPLIED BY ITS CLIENTS, AS WELL AS ITS CLIENTS’ PUBLICLY AVAILABLE INFORMATION AND PRESS RELEASES WHICH IT BELIEVES TO BE RELIABLE; HOWEVER, SUCH RELIABILITY CAN NOT BE GUARANTEED. INVESTORS SHOULD NOT RELY ON THE INFORMATION CONTAINED ON THIS WEBSITE. RATHER, INVESTORS SHOULD USE THE INFORMATION CONTAINED IN THIS WEBSITE AS A STARTING POINT FOR DOING ADDITIONAL INDEPENDENT RESEARCH ON THE FEATURED COMPANIES. THE ADVERTISEMENTS IN THIS WEBSITE ARE BELIEVED TO BE RELIABLE, HOWEVER, TRADIGITAL IR AND ITS OWNERS, AFFILIATES, SUBSIDIARIES, OFFICERS, DIRECTORS, REPRESENTATIVES, AND AGENTS DISCLAIM ANY LIABILITY AS TO THE COMPLETENESS OR ACCURACY OF THE INFORMATION CONTAINED IN ANY ADVERTISEMENT AND FOR ANY OMISSIONS OF MATERIALS FACTS FROM SUCH ADVERTISEMENT. TRADIGITAL IR IS NOT RESPONSIBLE FOR ANY CLAIMS MADE BY THE COMPANIES ADVERTISED HEREIN, NOR IS TRADIGITAL IR RESPONSIBLE FOR ANY OTHER PROMOTIONAL FIRM, ITS PROGRAM, OR ITS STRUCTURE. TRADIGITAL IR IS NOT AFFILIATED WITH ANY EXCHANGE, ELECTRONIC QUOTATION SYSTEM, THE SECURITIES EXCHANGE COMMISSION, OR FINRA.