This Innovative Biotech Company is Taking a Novel, Game-Changing Approach to Eradicating Many Respiratory Viruses Once and For All Including the Tripledemic Threat Of COVID, RSV, and the FLU!
NanoViricides, Inc. (NYSE American: NNVC) is a global leader in broad-spectrum antiviral nanomedicines developing drugs that work safely and effectively even against variants.
These drugs could be as revolutionary as antibiotics and do not rely on immune systems making them great for seniors and children!

Download The Corporate Presentation
NV-387 - A novel broad-spectrum antiviral
NanoViricides, Inc. (NYSE American: NNVC)’s lead drug candidate is NV-387 (drug product NV-CoV-2) for the treatment of RSV, COVID-19, Long COVID, Influenza, Bird Flu H5N1, and other respiratory viral infections.
Susceptible viruses CANNOT escape NV-387, even as they continue to evolve in the field, enabling NNVC to anticipate that nanoviricide drugs could have many decades long of “life of service.”
No matter how much the virus changes, it continues to use the same host-side signature to bind to and cause infection in the hosts, and thus the nanoviricide would be anticipated to continue to be effective.
Thus NV-387 and other antiviral drugs designed on the nanoviricides platform can be expected to have a long duration of effective usability against the target viruses, reaching into several decades, similar to antibiotics, but in stark contrast with current antiviral approaches.
An antiviral drug such as NV-387 if found to be effective in human clinical studies would be a highly desirable drug globally. It would enable treatment of patient as soon as they present to the physician with a viral disease without waiting for a test for identifying which viral infection it is.
This is reminiscent of how antibiotics are prescribed, without specific infectious agent identification, relying on the ultra-broad-spectrum of antibacterial activity.
Extremely Broad Antiviral Spectrum of NV-387 Resulted Because Many Diverse Viruses Use Common Host-Side Landing Sites
Over 90% of human pathogenic viruses are known to use one or more "landing sites" that are in the Sulfated Proteoglycans ("SPG") family. A successful host-mimetic nanoviricide drug using SPG as the key feature to attract viruses could theoretically be able to attack most if not all such viruses.
NV-387 is designed to mimic SPG and attack the virus as a cell-mimicking decoy. We have accumulated substantial evidence that in lethal viral infection animal studies, NV-387 demonstrated strong antiviral activity against a range of different virus families, exceeding or matching the activity of known approved drug agents.
Superior to Other Treatments
NV-387 was substantially superior to remdesivir in coronavirus infections, using a model for SARS-CoV-2 (COVID) virus, as reported earlier. We believe that NV-387 continues to be one of the most active antiviral drugs against multiple coronaviruses, and that it is a viable clinical candidate for drug development to treat COVID, Long COVID, as well as potentially MERS, SARS, and seasonal coronavirus infections.
NV-387 was substantially superior to the three approved drugs, namely Tamiflu®, Rapivab® , and Xofluza® against an Influenza H3N2 lethal lung viral infection study, as previously reported. We believe that NV-387 is expected to possess strong antiviral activity against H5N1 "Bird Flu" as well, given that H5N1 viruses are known to bind to heparan sulfate proteoglycans, and based on the observed broad-spectrum activity of NV-387.
NV-387 was at least as good as TPOXX® in a model animal study for the development of Smallpox/Mpox drugs, in two different ways of acquiring infection, as reported earlier.
NNVC has also found that NV-387 is capable of completely curing a lethal RSV lung virus infection in animals, leading to indefinite survival of the animals, as reported recently. There is no cure for RSV, and no approved drug for treatment of RSV infection other than the toxic last-resort drug ribavirin.
Moreover, even novel viruses, whether from natural sources or bio-engineered, are expected to be susceptible to NV-387 if they employ SPG for gaining access to human cells to infect and cause disease. Thus, NV-387 could be highly valuable for preparedness against novel viral epidemics and pandemics.
NV-387 could thus be a single drug to treat all of the "tripledemic" viruses, and more, when so approved!
"Resistance is Futile" - Host-Mimetic Technology To Solve the Greatest Pain for Antiviral Medicines, i.e. Virus Escape
No matter how much a virus changes in the field, it uses the same "landing sites" in the host body to gain access to cells, attach to and then fuse into the cells, causing an infection. A drug using the same landing sites and acting as a "decoy" would remain effective against the virus even as the virus changes, because the host side does not change.
NV-387 is designed to employ such advanced "host-mimetic" technology, built into the nanoviricide™ nanomedicine that is further designed to "look like a cell" to the virus.
In contrast, vaccines, antibodies and small chemical drugs all lose their effectiveness as the virus changes in the field. The virus is constantly changing due to various natural mechanisms such as mutations, recombinations, and/or re-assortments that are native to the virus lifecycle.
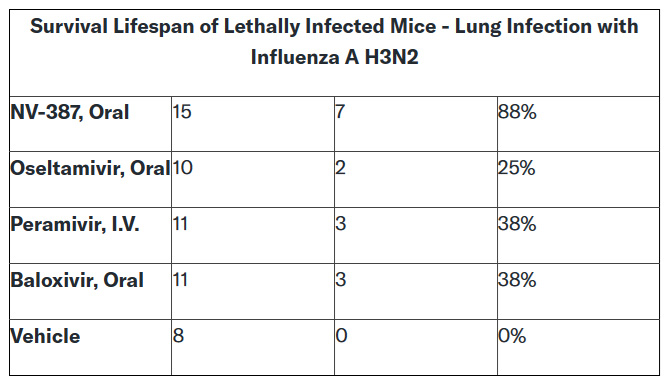
Given the broad spectrum of antiviral activity of NV-387 against viruses in many different virus families, NNVC believes that its effectiveness against Influenza A/H3N2 is indicative of potential antiviral activity against most if not all Influenza A viruses.
Ultra-Broad-Spectrum Antiviral Nanomedicines Enabled by the Nanoviricides Platform Would Open Up Very Large Market Sizes
Even with a decline since 2022, COVID-19 continues to hospitalize and kill people in the USA – the CDC website states 69,200 hospitalizations and 2,652 deaths since January 1, 2024; the worldwide market size for COVID-19 therapeutics is expected to exceed $16.2 Billion in 2031*.
*Source: Transparency Market Research
The market size for RSV therapeutics was estimated to be $2 Billion in 2023 and is expected to rise to exceed $8.5 Billion by the year 2031**.
**Source: Growth+ Market Reports.
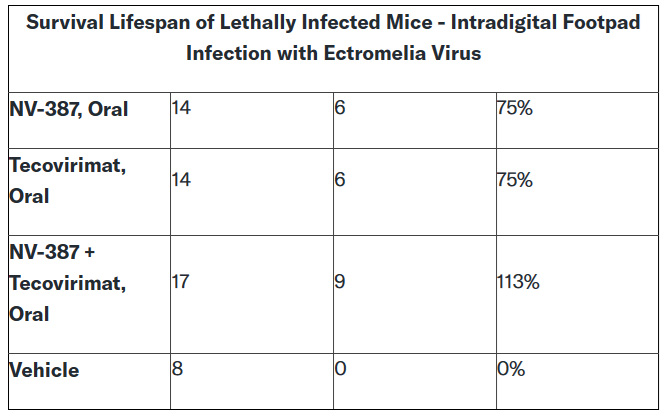
Previously, on November 14, 2023, we have reported that in a lethal intradigital footpad infection of mice with ectromelia virus, oral NV-387 treatment led to lifespan improvement comparable to oral tecovirimat treatment, with both treatments resulting in 14 days survival, whereas vehicle treated animals died in 8 days. Moreover, combined treatment with both NV-387 and tecovirimat resulted in a significantly improved survival of 17 days in this study.

Tecovirimat is the drug approved for smallpox under "animal rule" and is stockpiled by the Biomedical Advanced Research and Development Authority (BARDA). It was mobilized from the stockpile during the recent MPox Clade 2 pandemic.
BARDA is interested in development of additional poxvirus therapeutics as per a recent Broad-Agency Announcement (BAA). There is significant interest in the development of a smallpox therapeutic that works well by itself, as well as in combination with the known drug, tecovirimat. Tecovirimat has a low barrier of escape; a single mutation in one protein can enable the virus to escape this drug, adding to the significance of additional smallpox drug development.
NNVC believes that NV-387 is a viable clinical candidate to be developed by itself for the treatment of poxvirus infections under the US FDA "Animal Rule". In addition, the company believes that the combination of NV-387 and tecovirimat could reduce the potential for escape resistant generation against tecovirimat, as is known with other drug combination studies against viruses.
A safe and effective antiviral drug that the virus would not escape by simple mutations or field evolution is the holy grail of antiviral drug development!
Nanoviricides, Inc. is close to having a single drug NV-387 for the treatment of all of the tripledemic respiratory viruses - Coronaviruses, RSV, and Influenza A, which would be a revolutionary achievement!

Learn More about Nanoviricides, Inc. by gaining access to the latest corporate presentation
Download Research ReportWhat’s in store for biotech in 2024? Perhaps a lot of good!
As biotech stages a big comeback, NanoViricides, Inc. (NYSE American: NNVC) looks well-positioned to be a market disruptor with nontoxic, effective antiviral therapies based on patented nanomedicine technology.
NanoViricides, Inc. (NYSE American: NNVC) is a global leader in the application of nanomedicine technologies to the safe and effective treatment of viruses and their variants INCLUDING drugs against Covid-19, RSV and other respiratory viruses!
Even with a decline since 2022, COVID-19 continues to hospitalize and kill people in the USA – the CDC website states 69,200 hospitalizations and 2,652 deaths since January 1, 2024; the worldwide market size for COVID-19 therapeutics is expected to exceed $16.2 Billion in 2031*.
*Source: Transparency Market Research
The market size for RSV therapeutics was estimated to be $2 Billion in 2023 and is expected to rise to exceed $8.5 Billion by the year 2031**.
**Source: Growth+ Market Reports.
Analysts have said that the 2023 year-end spurt of biopharma mergers and acquisitions bodes well for the sector this year. The SPDR S&P Biotech ETF, which tracks the sector, had a strong November and December.
The recent AbbVie-ImmunoGen deal “may be the start of more consistent activity we will see in 2024,” RBC Capital Markets analyst Brian Abrahams wrote in a note published late recently. “We do believe we are likely past the XBI bottom and can look to somewhat improved enthusiasm for the group—in part driven by M&A—into 2024.”
AbbVie spent about $18.8 billion on two large deals in a matter of seven days.
According to an RBC Capital, the cumulative value of biopharma deals in 2023 has been $128 billion, up from $61 billion in 2022. Major acquisitions announced include Pfizer’s $43 billion deal for the cancer-focused biotech Seagen, and Merck’s $10.8 billion deal for the immunology-focused biotech Prometheus Biosciences.
RECENT COMPANY HIGHLIGHTS:
- Broad spectrum antiviral NV-387 showing promise against many virus families including and beyond Tripledemic (i.e. COVID-19, RSV, FLU)
- No adverse events in Phase I SAD and MAD studies even at the highest dose 40mg/Kg
- Found to be non-immunogenic, non-mutagenic, non-allergenic, and non-phototoxic.
- Strong safety allows use in pediatrics, adults with co-morbidities, and immune-compromised patients unlike limitations of products currently in the market.
- Developed Oral Syrup and Oral Gummies (soft solids) - good for geriatric and pediatric patients
- Drugs expected to continue to be effective even as the virus generates variants - unique receptor site doesn’t change
- Technology mimics unique receptor site used by virus; delivers to specific targets using receptor-recognition (no bulky antibodies)
- NanoViricides Bolsters Partnership Efforts - Engages Aagami Inc, which provides a significant boost to NNVC licensing and partnering efforts, while at the same time substantially freeing up our executives and staff to focus more strongly on further development of our multiple drugs and the nanoviricides platform through clinical trials
ANTIBODIES AND VACCINES ARE OUTDATED: NanoViricides, Inc. has a more innovative approach that works even when viruses mutate!!!
NANOVIRICIDES are better because they destroy viruses and their variants without relying on the patient's immune system, thereby making them effective for populations that include geriatric and pediatric patients.
- Antibodies only bind by two points to the virus, and destruction of the complex requires effective immune function, which is not the case in sick patients..
- Vaccines only train the body into producing antibodies against the virus in the vaccine. Antibodies and vaccines are easily overcome by viruses by mutating in the field, hence the need for annual influenza vaccine updates.
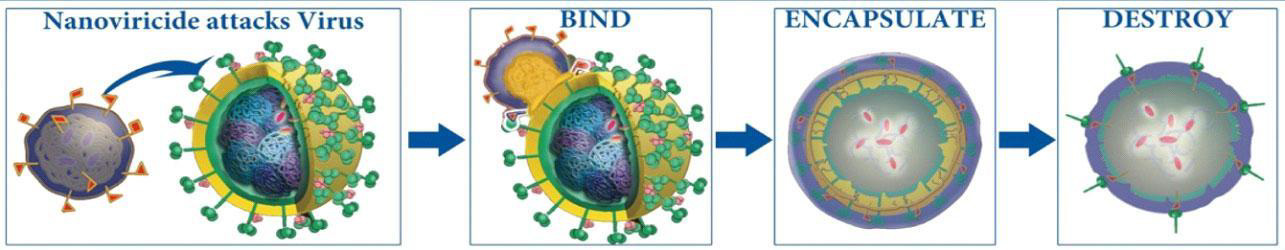
See Below for Nanoviricides' Unique, Novel, Post-Immunotherapeutic “Bind-Encapsulate-Destroy” Mechanism
NV 387 IN CLINICAL TRIALS
The drug, developed in response to the COVID-19 pandemic, demonstrated exceptional safety in clinical trials, even at the highest dose levels, with NO adverse events reported. The unique mechanism of action involves mimicking a cell membrane, encapsulating and blocking the virus.
Beyond COVID-19, the drug also displayed promising results against respiratory syncytial virus (RSV), offering a potential solution for infants and seniors where existing treatments like ribavirin may be contraindicated due to side effects.
The clinical trials involved both oral tablets and oral gummies, catering to various age groups, including pediatrics. NV 387 exhibited broad-spectrum activity against multiple strains of coronaviruses, showcasing effectiveness 10 times greater than remdesivir in preclinical studies.
The ongoing clinical trial progress and positive results position NanoViricides at the forefront of antiviral drug development, marking a significant milestone in their journey from preclinical research since 2005 to clinical trials.
The company believes that NV-387 not only binds to the virus, but fuses with the virus surface, uprooting the glycoproteins that are required for the virus to bind to the human cell (for example, the S protein, and its products S1 and S2 proteins from coronaviruses), thereby rendering the virus incapable of infecting a cell. In contrast, antibodies are only capable of covering the virus, generally incompletely, and require immune system assistance for clearing the resulting complex!
NV-387 HAS SHOWN BROAD-SPECTRUM ANTIVIRAL ACTIVITY ACROSS MULTIPLE VIRUS FAMILIES:
- NV-387 was found to be as effective as ribavirin, the toxic last resort drug, against RSV infection in a lethal lung infection animal model, as reported previously. RSV is a virus particularly threatening to vulnerable infants, young children, older adults, and immunocompromised populations.
- There is no approved drug for the treatment of RSV infection, except the toxic drug ribavirin which is only indicated for very severe cases due to its severe hemotoxicity. Ribavirin, a highly toxic drug that attacks red blood cells and can cause multi-organ failures is reserved for use as a last resort only in extremely severe hospitalized cases of RSV. NV-387 was almost as active as ribavirin.
- RSV is an important pathogenic virus that can cause lethal infection in infants as well as seniors and immunocompromised persons.
- Two different vaccines against RSV have been recently approved by the US FDA, but none are for pediatric use at present. Two different antibodies have been approved as prophylactic (i.e. to prevent RSV infection) for babies, but are not approved as therapeutics (i.e. after disease occurs).
- There is no safe and effective therapeutic available for RSV. Additionally, NV-387 has been found to be extremely safe in pre-clinical studies. Thus, we believe NV-387 could be a clinical quality drug candidate for the treatment of RSV infections.
- NV-387 was as effective as the approved drug tecovitrimat (TPOXX®, SIGA), in a lethal intra-digital infection by ectromelia virus in mice. Importantly, a combined drug made from NV-387 and tecovirimat was more effective than either drug alone, indicating NV-387 "plays well" with tecovirimat and acts by a different mechanism.
- Smallpox poses a significant biodefense threat. Ectromelia virus is a native virus of mice in the poxvirus family and is one of the key animal model viruses for developing smallpox therapeutics. Tecovirimat is an approved drug for treating smallpox infection based on the FDA "Animal Rule", and is stockpiled by the US "Strategic National Stockpile". It was mobilized during the recent monkeypox epidemic.
- It is important to develop additional smallpox therapeutics that work well with tecovirimat and by themselves, since viruses pose the threat of drug escape by mutation; further, in a bio-terrorism scenario, a human-engineered smallpox virus resistant to existing drugs could be a potential threat.

NV-387 ACTS BY A NOVEL MECHANISM:
NNVC developed NV-387 in response to the COVID pandemic as a broad-spectrum, pan-coronavirus antiviral. It is designed to "look like a cell" to the virus, displaying copious amounts of sites to which the virus binds on the surface of the nanoviricide nanomicelle, to trap and destroy the virus particle, rendering the virus incapable of infecting another cell.
The company calls this novel antiviral mechanism "Re-Infection Blocker".
In particular, NV-387 was designed to emulate an "attachment receptor family" called sulfated proteoglycans (S-PG), that over 90% of human pathogenic viruses are known to use for infecting cells. Therefore, in addition to Coronaviruses, RSV and Smallpox, the company anticipates that NV-387 may have effectiveness against many other viruses.
NNVC plans on continuing to study the antiviral spectrum of NV-387 with a view to expand its applications.
NV-387 could usher in a new era in the treatment of antiviral infections, if it is found to be broadly effective against additional viruses that use S-PG, evoking a comparison to how antibiotics changed the treatment of bacterial infections.
NV-387 ADDRESSES AN UNMET MEDICAL NEED FOR BROAD-SPECTRUM, SAFE AND EFFECTIVE ANTIVIRAL DRUG THAT WORKS AGAINST MULTIPLE VIRAL THREATS:
There is a significant unmet medical need for a broad-spectrum antiviral drug that is effective and useable in all segments of the population. There are substantial limitations for all currently approved COVID drugs in terms of both the eligibility of a COVID patient, and the effectiveness of the drug.
NNVC believes that the excellent safety and the distinctly different mechanism of NV-CoV-2 (NV-387) support the use of this drug across all patient populations. This is an important characteristic for a COVID drug as well as for a drug to treat RSV infection.
NV-387 ADDRESSES LARGE MARKET SIZES:
Even with a decline since 2022, COVID-19 continues to hospitalize and kill people in the USA – the CDC website states 69,200 hospitalizations and 2,652 deaths since January 1, 2024; the worldwide market size for COVID-19 therapeutics is expected to exceed $16.2 Billion in 2031*.
*Source: Transparency Market Research
The market size for RSV therapeutics was estimated to be $2 Billion in 2023 and is expected to rise to exceed $8.5 Billion by the year 2031**.
**Source: Growth+ Market Reports.
NANOVIRICIDES TECHNOLOGY WILL TRANSFORM THE WAY VIRUSES & THEIR VARIANTS ARE TREATED WORLDWIDE!
NNVC’s novel approach has already enabled variant-proof drugs, blocking the complete viral life cycle without requiring help from the host’s defense systems! If both the viral re-infection cycle, and viral replication cycle arms of the viral lifecycle are blocked, a cure for many viral diseases is possible!!
The Company’s virus-specific nanoviricides have been created against important viruses such as HIV, Influenza and Bird Flu by choosing highly virus-specific ligands.
Broad-spectrum nanoviricides have been created that can bind to possibly as many as 90-95% of known viruses. The Company is also developing broad-spectrum nanoviricides to combat several neglected tropical diseases, such as Dengue, Rabies, and Ebola/Marburg.
With its ongoing expansion and development of their diverse and promising product pipeline, NanoViricides, Inc. is positioned to be a dominant and innovative leader in the nanomedicine and antiviral therapy space.
Led by the inventor of nanoviricides technology President, Dr. Anil Diwan, and managed by a highly effective team with decades of entrepreneurial, pharmaceutical, and nanomedicine experience, Nanoviricides, Inc. has been able to keep both administrative and R&D costs at extremely low levels while expanding the drug pipeline.
LEADERSHIP
Creator and developer of nanomedicine technologies, Dr. Anil R. Diwan co-founded NanoViricides and currently serves as the company’s President and Executive Chairman. Diwan has over 35+ years of experience in entrepreneurship and bio-pharmaceutical R&D, including the invention of novel polymeric micelle-based nanomedicine technologies in 1991, the birth of NanoViricides, Inc in 2005, the uplisting from OTC to NYSE-mkt in 2013, and now the first clinical trials of its COVID-19 drug in 2023. Along the way, Dr. Diwan has led several of the finance efforts and has raised over 60+ MM in equity financing and has over 60 patents issued internationally resulting from three fundamental international patent applications.
Before that, he has won several NIH SBIR (Small business innovation research) grant awards and holds a Ph.D. from Rice University, TX, a B.Tech. from Indian Institute of Technology, Mumbai (IIT-B), India where he held high scholastic ranks.


THE BOTTOM LINE
NV-387 Could Revolutionize Antiviral Treatment Just as Antibiotics Did Against Bacteria!
There is no denying that the world is changing… viruses are becoming more rampant. NNVC, an exciting NYSE company that is ahead of the problem, could see unstoppable growth ahead!
As biotech stages a big comeback, one NYSE-traded company looks well-positioned to be a market disruptor with nontoxic, effective antiviral therapies based on patented nanomedicine technology.
This global leader in broad-spectrum antiviral nanomedicines had remarkable news recently, revealing that the antiviral activity of its NV-387 oral treatment against RSV/A2 is strong enough to have resulted in full survival of lethally infected animals. The company believes the NV-387 oral treatment is capable of curing RSV infection. Why is this important? Because there is currently no approved treatment for RSV other than ribavirin. A safe and effective treatment remains an unmet medical need!
The company has sufficient funds to complete the on-going human clinical trials for its lead drug candidate NV-CoV-2 which is the drug product based on its "nanoviricide" active pharmaceutical ingredient ("API"), NV-387.
The successes of NV-387 as a broad-spectrum antiviral bode well for validating the multiple modalities in which NNVC’s Nanoviricides Platform Technology can be employed to revolutionize the treatment of viral infections as well as pandemic preparedness response.
This year the company has a focus on advancing NV-387 through further clinical trials, expanding its treatment capabilities to address unmet medical needs. This includes RSV, the flu, and other viruses.
NanoViricides, Inc.’s innovative platform technology, R&D agility, flexible cGMP compliant facility, highly effective management team, and extensive anti-viral experience should place this company at the top of your “invest” watchlist!

Learn More about Nanoviricides, Inc. by gaining access to the latest corporate presentation
Download PRESENTATIONTHIS IS A PAID ADVERTISEMENT
NO INVESTMENT ADVICE
SCD Media LLC (d/b/a “Smallcaps Daily”), hereinafter referred to as “Smallcaps Daily,” and their affiliates and control persons (the “Publisher”) are in the business of publishing favorable information and/or advertisements (the “Information”) about the securities of publicly traded companies (each an “Issuer” or collectively the “Issuers”) in exchange for compensation (the “Campaigns”). Persons receiving the Information are referred to as the “Recipients.” The person or entity paying the Publisher for the Campaign is referred to herein as the “Paying Party”. The Paying Party may be an Issuer, an affiliated or non-affiliate shareholder of an Issuer, or another person hired by the Issuer or an affiliate or non-affiliate shareholder of the Issuer. The nature and amount of compensation paid to the Publisher for the Campaign and creating and/or publishing the Information about each Issuer is set forth below under the heading captioned, “Compensation”.
This website provides information about the stock market and other investments. This website does not provide investment advice and should not be used as a replacement for investment advice from a qualified professional. This website is for informational purposes only. The Author of this website is not a registered investment advisor and does not offer investment advice. You, the reader, bear responsibility for your own investment decisions and should seek the advice of a qualified securities professional before making any investment.
Nothing on this website should be considered personalized financial advice. Any investments recommended herein should be made only after consulting with your personal investment advisor and only after performing your own research and due diligence, including reviewing the prospectus or financial statements of the issuer of any security.
Smallcaps Daily, its managers, its employees, affiliates, and assigns (collectively the "Publisher") do not make any guarantee or warranty about the advice provided on this website or what is otherwise advertised above.
Release of Liability: through use of this website, viewing or using you agree to hold Smallcaps Daily, its operators, owners, and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources that we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. Smallcaps Daily encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the company profiled or is available from public sources and Smallcaps Daily makes no representations, warranties, or guarantees as to the accuracy or completeness of the disclosure by the profiled company. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provided herein. Instead, Smallcaps Daily strongly urges you to conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Smallcaps Daily’s full disclosure is to be read and fully understood before using Smallcaps Daily's website, or joining Smallcaps Daily's email or text list. From time to time, Smallcaps Daily will disseminate information about a company via website, email, sms, and other points of media. By viewing Smallcaps Daily's website and/or reading Smallcaps Daily's email or text newsletter you are agreeing to this ----> https://SmallcapsDaily.com/disclaimer/. All potential percentage gains discussed in any communications are based on calculations from the low to the high of the day. We are engaged in the business of marketing and advertising companies for monetary compensation.
If you have questions or concerns about a product you’ve seen in one of our emails, emails, text newsletters or SMS, we encourage you to reach out to that company directly.
Disclaimer – Always do your own research and consult with a licensed investment professional before investing. This communication is never to be used as the basis of making investment decisions and is for entertainment purposes only. At most, this communication should serve only as a starting point to do your own research and consult with a licensed professional regarding the companies profiled and discussed. Conduct your own research. This newsletter is a paid advertisement, not a recommendation nor an offer to buy or sell securities. This newsletter is owned, operated, and edited by the owner of Smallcaps Daily. Any wording found in this e-mail or disclaimer referencing to “I” or “we” or “our” refers to Smallcaps Daily. Our business model is to be financially compensated to market and promote small public companies. By reading our newsletter and our website you agree to the terms of our disclaimer, which are subject to change at any time. We are not registered or licensed in any jurisdiction whatsoever to provide investing advice or anything of an advisory or consultancy nature and are therefore unqualified to give investment recommendations. Companies with low prices per share are speculative and carry a high degree of risk, so only invest what you can afford to lose. By using our service, you agree not to hold our site, its editors, owners, or staff liable for any damages, financial or otherwise, that may occur due to any action you may take based on the information contained within our newsletters or on our website. We do not advise any reader to take any specific action. Losses can be larger than expected if the company experiences any problems with liquidity or wide spreads. Our website and newsletter are for entertainment purposes only. Never invest purely based on our alerts. Gains mentioned in our newsletter and on our website may be based on end-of-day or intraday data. This publication and its owners and affiliates may hold positions in the securities mentioned in our alerts, which we may sell at any time without notice to our subscribers, which may have a negative impact on share prices. If we own any shares, we will list the information relevant to the stock and the number of shares here.
COMPENSATION
In compliance with section 17(b) of the Securities Act we are disclosing that we have been compensated a fee pursuant to an agreement between Smallcaps Daily and IA Media LLC (d/b/a/ “IA Media”) hereinafter referred to as IA Media. Small Caps Daily was hired by IA Media for a period beginning January 2024 and ending January 2024 to publicly disseminate information about NanoViricides, Inc., via website, email, and SMS. We were paid up to twenty-five thousand usd via ACH. Subsequently, Small Caps Daily was hired by IA Media for a period beginning February 2024 and ending February 2024 to publicly disseminate information about NanoViricides, Inc., via website, email, and SMS. We were paid up to twenty-five thousand usd via ACH. Readers are advised to review SEC periodic reports: forms 10Q 10K, form 8K, insider reports, forms 3, 4, 5 schedule 13d. Smallcaps Daily is compliant with the CAN-SPAM Act of 2003. Smallcaps Daily does not offer investment advice or analysis, and Smallcaps Daily further urges you to consult your own independent tax, business, financial, and investment advisors. investing in micro-cap, small-cap, and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor's investment may be lost or impaired due to the speculative nature of the companies profiled. The private securities litigation reform act of 1995 provides investors a safe harbor in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events, or performance are not statements of historical fact but may be forward-looking statements. Forward-looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward-looking statements in this action may be identified through the use of words such as projects, foresee, expects, will, anticipates, estimates, believes, understands, or that by statements indicating certain actions & quotes; may, could, or might occur. Understand there is no guarantee past performance will be indicative of future results in preparing this publication. Smallcaps Daily has relied upon information supplied by its clients, as well as its clients’ publicly available information and press releases which it believes to be reliable; however, such reliability can not be guaranteed. Investors should not rely on the information contained on this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, Smallcaps Daily and its owners, affiliates, subsidiaries, officers, directors, representatives, and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of material facts from such advertisement. Smallcaps Daily is not responsible for any claims made by the companies advertised herein, nor is Smallcaps Daily responsible for any other promotional firm, its program, or its structure. Smallcaps Daily is not affiliated with any exchange, electronic quotation system, the Securities Exchange Commission, or FINRA.
Copyright © 2024 Smallcaps Daily. All rights reserved.
