There’s a good reason why an insider has significantly increased his holdings in this rising biopharma company….

This ophthalmology-focused bio-pharma company is developing its game-changing OK-101 drug candidate to treat dry eye disease (DED) – a condition that addresses a significant unmet need in a multi-billion-dollar market!
Now may be a critical time to have the company on your radar as only weeks ago, the U.S. Food and Drug Administration (FDA) cleared OKYO’s Investigational New Drug (IND) to initiate a Phase 2, first-in-human, clinical study of OK-101 for the treatment of DED!


Download Research Report
Okyo Pharma Limited (NASDAQ: OKYO) (LSE: OKYO) is a quietly trading biopharma company that is leading the way in eye care advancements and is at the early stages of its growth story.
THE TOP 10 REASONS TO HAVE OKYO ON YOUR RADAR:
- OKYO’s immediate goal is the development of a first-in-class drug that combines both anti-inflammatory and ocular pain-reducing activity to treat dry eye disease (DED).
- Worldwide, 360 million patients suffer from DED. In the United States, 20 million patients suffer from this condition, with DED remaining a major unmet medical need.
- Currently, there is NO FDA approved topical treatment for ocular pain.
- In animal models of DED, OKYO’s lead drug candidate OK-101 was effective in suppressing ocular inflammation by downregulating key inflammatory CD4+ T cells and restoring the normal level of goblet cells responsible for mucin production.
- The company received FDA clearance in December 2022 for its Investigational New Drug (IND) application from the U.S. Food and Drug Administration (FDA) to initiate a Phase 2, first-in-human, clinical study of OK-101 for the treatment of dry eye disease (DED).
- Pre-IND meeting with FDA gave concurrence that its planned Phase 2 trial, if the primary clinical end points are hit, has potential to qualify as a Phase 3 trial!
- OKYO’s Phase 2 trial in 240 DED patients is planned to begin in 1Q 2023 with release of top-line data in 4Q 2023 – and with a successful outcome would qualify as a major demonstration the drug works!
- OKYO has patent protection until 2039!
- Should OKYO be successful with the results from the clinical trial in 4Q 2023, the company could be in a strong negotiating position to clinch a business development partnership or trade sale to Big Pharma.
- Last year Non-Executive Chairman Gabriele Marco Cerrone bought 9.9M shares on the LSE at a price per share of UK£0.06. Executives and board members are more confident about the direction of their company when they start buying up more shares.
“We are very pleased to receive clearance from the FDA to initiate our OK-101 Phase 2 study. We believe this first-in-human study will help demonstrate that OK-101 may provide a new way to treat DED patients who are not well-served by currently approved drugs. Based on earlier feedback from the FDA we are designating primary and secondary efficacy endpoints in this study that include both a sign and a symptom of the disease. Should our Phase 2 study meet its prespecified primary endpoint, it may accelerate the timeline to a new drug application (NDA) filing for OK-101 with the FDA. The clearance of our IND for OK-101 has been a key priority for the company this past year, and we are excited to be moving this drug into the clinic in the first quarter of 2023.”
Gary S. Jacob, Ph.D., Chief Executive Officer
Greetings Investors,
The healthcare arena is just what the doctor ordered for 2023 as inflation continues and a recession looms. Healthcare is one of the most stable industries, and people will continue to spend money on medicine regardless of how the economy is doing.
Additionally, the World Health Organization (WHO) has estimated that the number of people who are aged 60 and older will almost double by 2050! The market will reach a staggering 426 million people.
What does this mean? It means more prescription drugs will keep older people healthy and active as their bodies wear down.
As baby boomers age, eye issues continue to boom. As more people spend their time on screens, eye issues continue to accelerate.
The growing eye industry is something investors can’t ignore much longer.
Of your five senses, eyesight ranks right at the top. And when eyes are affected by various conditions, people will do what they can to alleviate their symptoms and improve their eye condition.
Dry eye disease is one of the most common ocular eye conditions. It’s no surprise that the market has become extensive for dry eye drugs including brand names like Novartis’ Xiidra and Allergan’s Restasis.
These drugs are expensive too. Xiidra is not usually covered by Medicare insurance plans and there is no generic alternative available for Xiidra. Restasis is a prescription eye drop that increases tear production to relieve chronic dry eye and can also cost hundreds of dollars.
Not to mention that both drugs come with side effects, have inadequate efficacy, and a slow onset of action.
Healthcare research for the eyes is laying the groundwork for drug technology that has never been seen before and treatments and therapies are becoming more sophisticated with every passing year.
Being conscious of which companies are successful in capitalizing on innovative ways to treat eye problems could be advantageous for any portfolio!
What makes OKYO stand out in the DED treatment space is a new chemical entity that targets BOTH ocular inflammation and corneal neuropathic pain in dry eye disease! Something that has yet to be seen for any other drug!
Such a drug profile potentially opens a wide door for OKYO to tap into an underserved niche market and creates the potential for shareholder growth.
Company Overview
OKYO Pharma Limited (Nasdaq: OKYO, LSE: OKYO) is a life sciences company developing next-generation therapeutics to improve the lives of patients suffering from inflammatory eye diseases and ocular pain.
OKYO’s research program is focused on a novel G Protein-Coupled Receptor (GPCR), which the company believes plays a key role in the pathology of these inflammatory eye diseases of high unmet medical need.
The company’s therapeutic approach is focused on targeting inflammatory and pain modulation pathways that drive these conditions.

OKYO is concentrating on the development of its drug candidate OK-101 to treat ocular diseases, including:
- Dry eye (DED) uveitis
- Uveitis
- Allergic conjunctivitis
- Ocular pain
The immediate focus of the company is the clinical development of OK-101 to treat dry eye disease (DED).
In November 2022 the company filed an IND to treat DED with the U.S. Food and Drug Administration and plans to skip the normal Phase 1 trials conducted in volunteers and go straight to the initiation of a Phase 2 trial in 240 DED patients in the first quarter of 2023. This is just around the corner!
The Problem
Your tears protect the surface of your eyes from damage. Damage to the corneal surface typically leads to ocular inflammation. If left untreated, severe ocular inflammation can lead to abrasion of the corneal surface, corneal ulcers and even vision loss.
Ocular pain can be caused by many issues including allergies, inflammation, headaches, contact lens problems, dry eyes, eyelid infections, and pink eye.

OK-101
OKYO’s OK-101 is a promising candidate for the treatment of both inflammation and ocular pain.
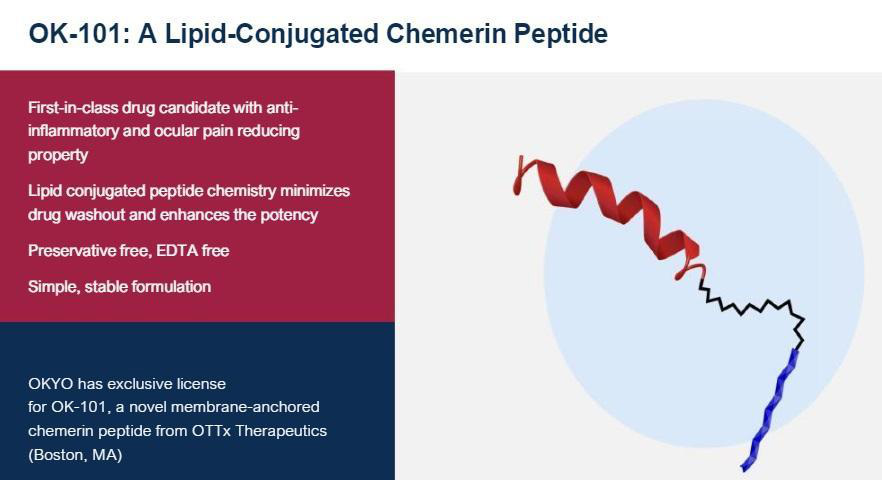
OK-101 is a lipid-conjugated chemerin peptide agonist of the ChemR23 G-protein coupled receptor class which is typically found on immune cells of the eye responsible for the inflammatory response.
ChemR23 receptor on leukocytes targeted by OK-101 is also expressed on neurons and glial cells in the dorsal root ganglion and spinal cord.
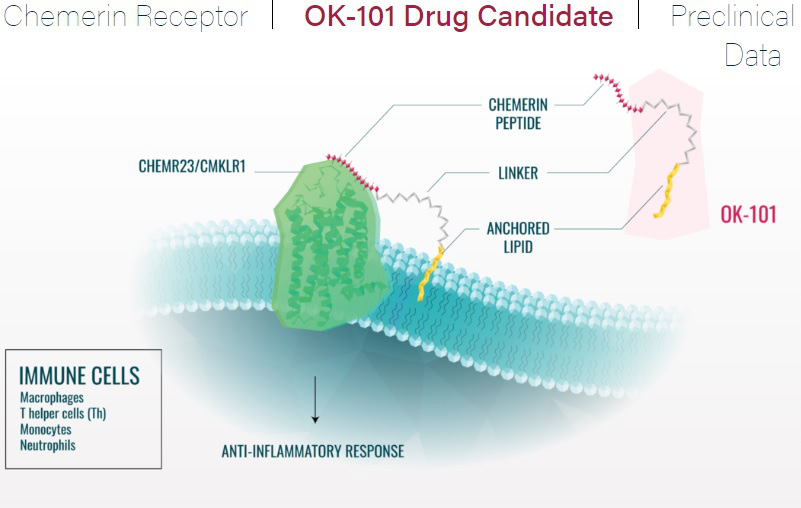
OK-101 has been shown to produce anti-inflammatory and neuropathic pain-reducing activities in mouse models of DED and corneal neuropathic pain, respectively, and is designed to combat washout through the inclusion of the lipid ‘anchor’ contained in the drug molecule to enhance the residence time of OK-101 within the ocular environment.
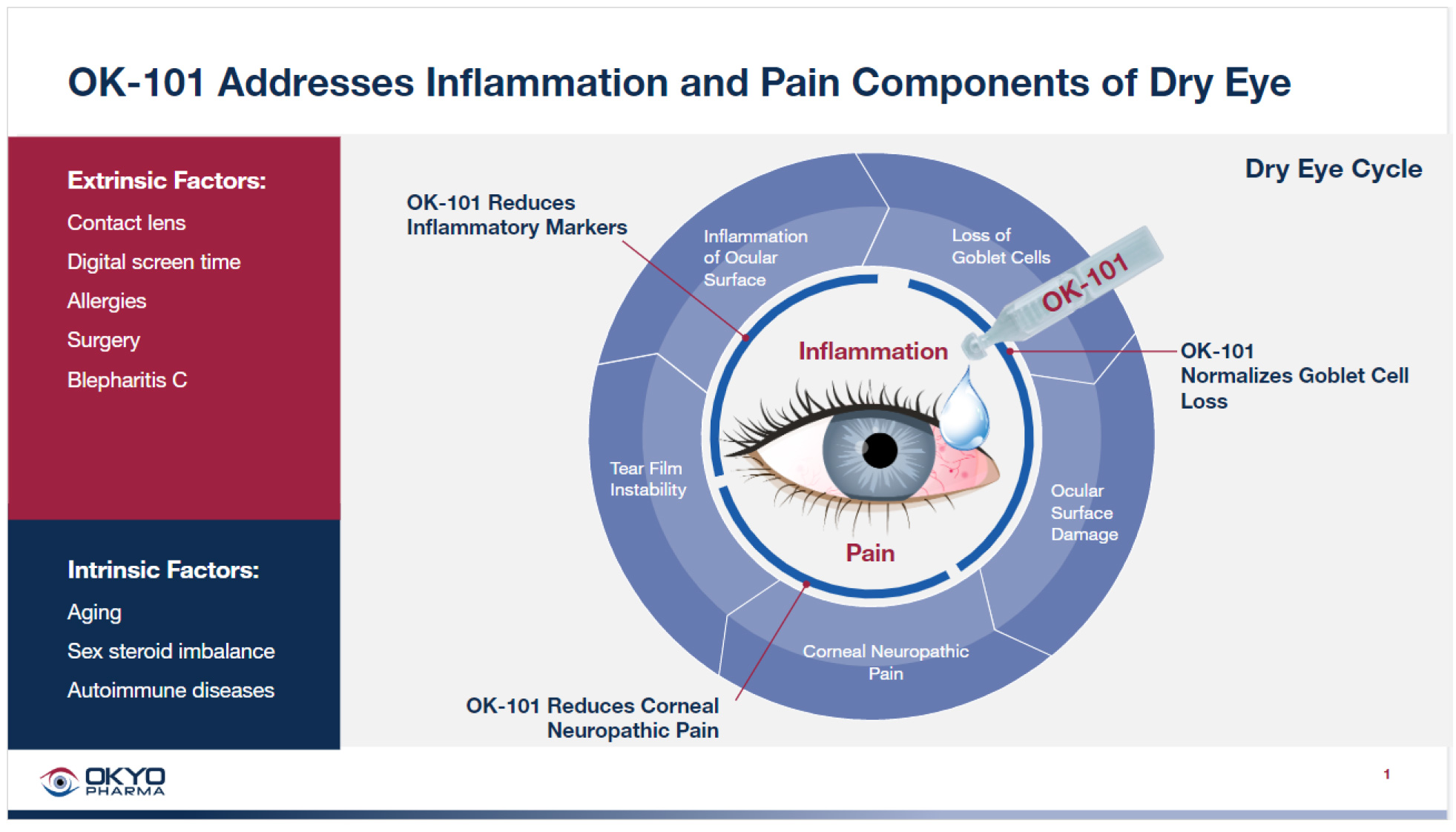
- One of the major challenges with topical administration of any drug designed for treating DED is drug wash-out through natural processes of tearing and blinking, minimizing drug ‘residence’ time at the ocular site needed to provide a pharmacologic benefit.
- Membrane Anchored Peptide (MAP) technology enabled the development of a long acting and stable OK-101 drug candidate with enhanced potency and the expectation of increased drug residence time on the ocular surface.
- OKYO’s lead drug candidate OK-101 consists of a 10-mer C-terminal chemerin peptide sequence, a linker component, and an anchoring lipid domain.
Dry Eye Disease
For those who have dry eye disease (DED), they know exactly why it’s an inconvenience, People suffering from dry eyes have difficulty maintaining a healthy social life. It can also significantly reduce performance at work and make it difficult to enjoy things that they like to do.
DED makes life difficult on multiple levels. In addition to having an impact on visual acuity, the condition is chronic - there is no cure, and individuals can suffer from pain, itchiness, burning sensations, and blurry vision that interferes with their quality of life.
The global dry eye syndrome market size was USD $5.22 billion in 2019 and is projected to reach USD $6.54 billion by 2027, exhibiting a CAGR of 4.7% during the forecast period.

Market Drivers
The increase in the prevalence of dry eye-related diseases may be attributed to a number of causes, including aging, a decline in supporting hormones, systemic inflammatory diseases, ocular surface diseases, or procedures that disrupt the cholinergic neurons that promote tear secretion.
According to a December 2021 article titled "Estimated Annual Economic Burden of Dry Eye Disease Based on a Multi-Center Analysis in China: A Retrospective Study," DED is now the fifth most common ocular condition in women and the ninth most common in men in the United States among those who need eye care.
OKYO’s focus is to suppress the inflammation and pain associated with the uveitis using our lead drug candidate OK-101.
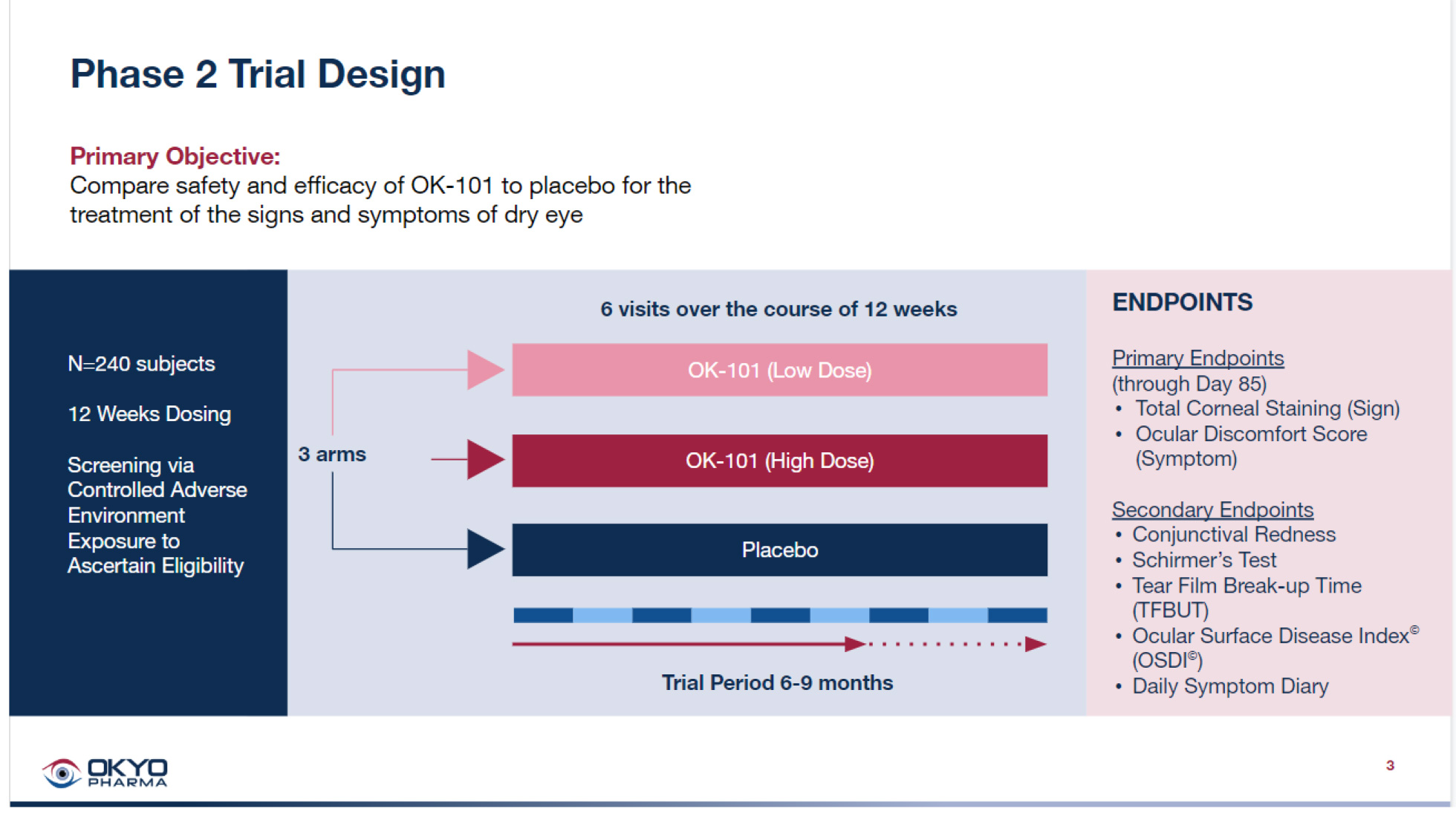
OK-101 CLINICAL PLAN
OKYO spent the last 18 months stepping through a multiplicity of pre-clinical activities, including drug manufacture and formulation, animal toxicity studies, DED animal model studies, analytics development, GMP drug manufacturing scale-up, pre-IND meeting with FDA, and the generation of the IND package for submission to FDA. This work was facilitated by a strong collaboration with OKYO’s premier clinical CRO, ORA Inc. which has considerable proven experience in conducting dry eye disease trials.
The Pre-IND meeting was successfully conducted in February 2022. Both nonclinical and clinical development milestones were covered in the meeting, facilitated by Ora Inc., with the FDA agreeing with the company’s plan to skip phase 1 trials, and providing written guidance on the proposed phase 2 trial in DED patients. FDA concurred with OKYO’s decision to designate co-primary efficacy endpoints covering both a sign and a symptom of dry disease in the clinical protocol of the trial.
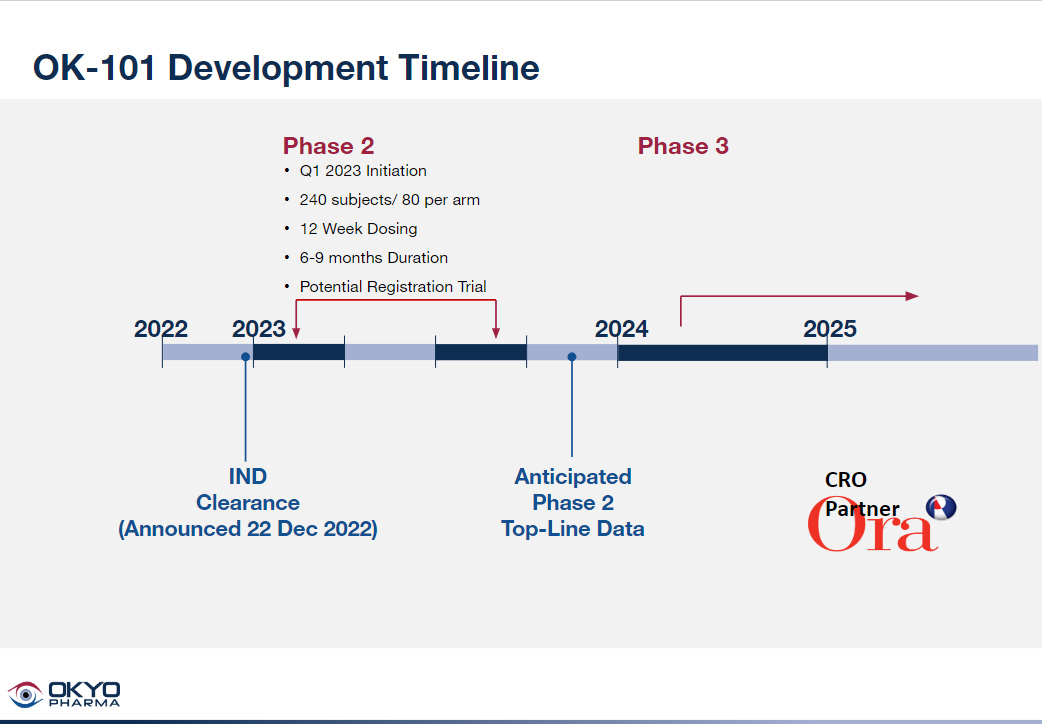
OKYO successfully obtained clearance from the FDA in December 2022 on its IND on OL-101 to treat DED patients and plans to open its phase 2 trial in 1Q 2023. The OK-101 timeline is shown below. What is noteworthy is the planned release of top-line data from the trial in 4Q 2023.
Ocular Pain
Approximately 5 million people suffer from ocular pain every year.
Neuropathic corneal pain, a severe, chronic, and debilitating disease for which there are no approved commercial treatments currently available.
Current treatments are limited to short term NSAIDs, steroids, and opioids in severe cases. Side effects and the risk of addiction to opioids is a serious concern.
The global ocular pain market is expected to reach half a billion dollars by 2030.
Topical administration of OKYO’s lead drug candidate OK-101 was effective in suppressing corneal pain in a ciliary nerve ligation mouse model of neuropathic corneal pain; exhibiting a potency similar to that of gabapentin, a commonly used oral drug for neuropathic pain that was conversely given by intraperitoneal injection.
The pain-relieving potential of a dry eye drug would provide an important benefit to the considerable number of dry eye patients suffering from ocular pain in addition to the existing inflammatory effects of the condition.
Leadership
Gary Jacob PhD - Chief Executive Officer/Director

Dr. Jacob has over 35 years of extensive experience in the pharmaceutical and biotechnology industries across multiple disciplines, including research and development, operations, business development, capital financing activities and senior management expertise.
He has developed broad and influential contacts throughout the biopharmaceutical, financial, banking and investor communities. Dr. Jacob is the Co-Founder and former CEO and Chairman of Synergy Pharmaceuticals. During his time at Synergy, he served as Chairman, Chief Executive Officer and Executive Chairman, and is the co-inventor of Synergy’s FDA-approved drug Trulance® which is currently marketed in the U.S. by Bausch Health, Inc. to treat functional GI disorders. Dr. Jacob is also the former CEO and Managing Director of Immuron Inc., an Australian biotechnology company dual-listed on the Australian ASX exchange and on NASDAQ. Dr. Jacob currently is Chairman of the Board of Hepion Pharmaceuticals, Inc., a public NASDAQ listed company with a drug in clinical development to treat nonalcoholic steatohepatitis (NASH), and is also on the Board of Directors of Cardiff Oncology, Inc., a NASDAQ listed public oncology company. He served as Chief Executive Officer and Director of Callisto Pharmaceuticals, Inc. from May 2003 until January 2013.
Prior to his involvement with Callisto and Synergy, Dr. Jacob was at Monsanto/G.D. Searle, where he was Director of Glycobiology and a Monsanto Science Fellow, specializing in the field of Glycobiology and drug discovery. Dr. Jacob holds over 30 patents and is the co-inventor of two pharmaceutical drugs which are FDA approved. Dr. Jacob earned a B.S. cum laude in Chemistry from the University of Missouri, St. Louis and holds a Ph.D. in Biochemistry from the University of Wisconsin, Madison.
Raj Patil, PhD - Chief Scientific Officer

Dr. Patil brings 30 years of ophthalmic experience and a powerful combination of academic scholarship and pharmaceutical R&D excellence.
Raj previously worked with Ora Inc, as Vice President of Research & Development, where he was responsible for driving all anterior and posterior segment research of Ora’s R&D Institute. Earlier in his career, he worked at iVeena Delivery Systems as Vice President of Advanced Ocular Delivery Systems. His tenure at iVeena included a two-year sabbatical in Singapore, where he served as an Associate Professor of Ophthalmology at DUKE/NUS Medical School and Principal Investigator at Singapore Eye Research Institute.
Raj also held a number of leadership roles at Alcon/Novartis Institute of Biomedical Research, including Associate Director of Research and Head of Molecular Pharmacology glaucoma and retina research. Prior to joining the business world, Dr. Patil served as an Associate Professor of Ophthalmology, Cell Biology & Genetics at University of Nebraska Medical Centre in Omaha and as an Assistant Professor of Ophthalmology, Molecular Biology & Pharmacology at Washington University in St. Louis.
Raj received his PhD in Biochemistry from National Chemical Laboratory/University of Pune, India and completed his postdoctoral training in Biochemistry and Molecular Biology at the University of Michigan, Ann Arbor, MI. He is the recipient of Olga Keith Wiess Special Scholar Award from Research to Prevent Blindness Foundation and NIH Director’s New Innovator Award. Dr. Patil has authored over 50 peer-reviewed research articles and serves as reviewer and editorial board member for numerous journals and is frequently invited to lecture at academic and industry events.
Keeren Shah - Chief Financial Officer

Keeren Shah serves as our Chief Financial Officer. Ms. Shah currently also serves as the Finance Director of Tiziana Life Sciences LTD, Accustem Sciences Limited and Rasna Therapeutics Inc., having previously served as the Group Financial Controller for all businesses from June 2016 to July 2020. Prior to joining the Company, Ms. Shah spent 10 years at Visa, Inc. as a Senior Leader in its finance team where she was responsible for key financial controller activities, financial planning and analysis, and core processes as well as leading and participating in key transformation programmes and Visa Inc.’s initial public offering. Before joining Visa, Ms. Shah also held a variety of finance positions at other leading companies including Arthur Andersen and BBC Worldwide. She holds a Bachelor of Arts with honours in Economics and is a member of the Chartered Institute of Management Accountants.
OKYO CEO Gary Jacob has told Proactive’s Andrew Scott it’s time to take the company from the development stage and into the clinic.

The Bottom Line
This is one of the most exciting times for Okyo Pharma Limited (NASDAQ: OKYO) (LSE: OKYO) as it moves toward initiating a Phase 2, first-in-human, clinical study of OK-101 for the treatment of DED.
OKYO’s clinical plan represents an ambitious rapid timeline in the clinic to attain clinical results validating that the drug works.
If OKYO is successful in hitting its clinical trial efficacy endpoints before the end of this year, the Company could be in a strong negotiating position to clinch a business development partnership and/or potentially a trade sale to a Big Pharma focused on eye diseases.
Start your research right away!

THIS IS A PAID ADVERTISEMENT
NO INVESTMENT ADVICE
SCD Media LLC (d/b/a “Smallcaps Daily”), hereinafter referred to as “Smallcaps Daily,” and their affiliates and control persons (the “Publisher”) are in the business of publishing favorable information and/or advertisements (the “Information”) about the securities of publicly traded companies (each an “Issuer” or collectively the “Issuers”) in exchange for compensation (the “Campaigns”). Persons receiving the Information are referred to as the “Recipients.” The person or entity paying the Publisher for the Campaign is referred to herein as the “Paying Party”. The Paying Party may be an Issuer, an affiliated or non-affiliate shareholder of an Issuer, or another person hired by the Issuer or an affiliate or non-affiliate shareholder of the Issuer. The nature and amount of compensation paid to the Publisher for the Campaign and creating and/or publishing the Information about each Issuer is set forth below under the heading captioned, “Compensation”.
This website provides information about the stock market and other investments. This website does not provide investment advice and should not be used as a replacement for investment advice from a qualified professional. This website is for informational purposes only. The Author of this website is not a registered investment advisor and does not offer investment advice. You, the reader, bear responsibility for your own investment decisions and should seek the advice of a qualified securities professional before making any investment.
Nothing on this website should be considered personalized financial advice. Any investments recommended herein should be made only after consulting with your personal investment advisor and only after performing your own research and due diligence, including reviewing the prospectus or financial statements of the issuer of any security.
Smallcaps Daily, its managers, its employees, affiliates, and assigns (collectively the "Publisher") do not make any guarantee or warranty about the advice provided on this website or what is otherwise advertised above.
Release of Liability: through use of this website, viewing or using you agree to hold Smallcaps Daily, its operators, owners, and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources that we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. Smallcaps Daily encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the company profiled or is available from public sources and Smallcaps Daily makes no representations, warranties, or guarantees as to the accuracy or completeness of the disclosure by the profiled company. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provided herein. Instead, Smallcaps Daily strongly urges you to conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Smallcaps Daily’s full disclosure is to be read and fully understood before using Smallcaps Daily's website, or joining Smallcaps Daily's email or text list. From time to time, Smallcaps Daily will disseminate information about a company via website, email, sms, and other points of media. By viewing Smallcaps Daily's website and/or reading Smallcaps Daily's email or text newsletter you are agreeing to this ----> https://Smallcaps Daily.com/disclaimer/. All potential percentage gains discussed in any communications are based on calculations from the low to the high of the day. We are engaged in the business of marketing and advertising companies for monetary compensation.
If you have questions or concerns about a product you’ve seen in one of our emails, emails, text newsletters or SMS, we encourage you to reach out to that company directly.
Disclaimer – Always do your own research and consult with a licensed investment professional before investing. This communication is never to be used as the basis of making investment decisions and is for entertainment purposes only. At most, this communication should serve only as a starting point to do your own research and consult with a licensed professional regarding the companies profiled and discussed. Conduct your own research. This newsletter is a paid advertisement, not a recommendation nor an offer to buy or sell securities. This newsletter is owned, operated, and edited by the owner of Smallcaps Daily. Any wording found in this e-mail or disclaimer referencing to “I” or “we” or “our” refers to Smallcaps Daily. Our business model is to be financially compensated to market and promote small public companies. By reading our newsletter and our website you agree to the terms of our disclaimer, which are subject to change at any time. We are not registered or licensed in any jurisdiction whatsoever to provide investing advice or anything of an advisory or consultancy nature and are therefore unqualified to give investment recommendations. Companies with low prices per share are speculative and carry a high degree of risk, so only invest what you can afford to lose. By using our service, you agree not to hold our site, its editors, owners, or staff liable for any damages, financial or otherwise, that may occur due to any action you may take based on the information contained within our newsletters or on our website. We do not advise any reader to take any specific action. Losses can be larger than expected if the company experiences any problems with liquidity or wide spreads. Our website and newsletter are for entertainment purposes only. Never invest purely based on our alerts. Gains mentioned in our newsletter and on our website may be based on end-of-day or intraday data. This publication and its owners and affiliates may hold positions in the securities mentioned in our alerts, which we may sell at any time without notice to our subscribers, which may have a negative impact on share prices. If we own any shares, we will list the information relevant to the stock and the number of shares here.
COMPENSATION
In compliance with section 17(b) of the Securities Act we are disclosing that we have been compensated a fee pursuant to an agreement between Smallcaps Daily and TraDigital Marketing Group, Inc. (d/b/a/ “TraDigital IR”) hereinafter referred to as TraDigital IR. Please see TraDigital IR’s disclosure page here. SmallCaps Daily was hired by TraDigital IR for a period beginning February 2023 and ending April 2023 to publicly disseminate information about OKYO Pharma Limited via website, email, and sms. We were paid five thousand usd via ACH. Subsequently, SmallCaps Daily was hired by TraDigital IR for a period beginning August 25, 2023 and ending August 28, 2023 to publicly disseminate information about OKYO Pharma Limited via website, email, and sms. We were paid up to twenty-five thousand usd via ACH. Subsequently, SmallCaps Daily was hired by TraDigital IR for a period beginning October 02, 2023 and ending October 05, 2023 to publicly disseminate information about OKYO Pharma Limited via website, email, and sms. We were paid up to twenty-five thousand usd via ACH. Readers are advised to review SEC periodic reports: forms 10Q 10K, form 8K, insider reports, forms 3, 4, 5 schedule 13d. Smallcaps Daily is compliant with the CAN-SPAM Act of 2003. Smallcaps Daily does not offer investment advice or analysis, and Smallcaps Daily further urges you to consult your own independent tax, business, financial, and investment advisors. investing in micro-cap, small-cap, and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor's investment may be lost or impaired due to the speculative nature of the companies profiled. The private securities litigation reform act of 1995 provides investors a safe harbor in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events, or performance are not statements of historical fact but may be forward-looking statements. Forward-looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward-looking statements in this action may be identified through the use of words such as projects, foresee, expects, will, anticipates, estimates, believes, understands, or that by statements indicating certain actions & quotes; may, could, or might occur. Understand there is no guarantee past performance will be indicative of future results in preparing this publication. Smallcaps Daily has relied upon information supplied by its clients, as well as its clients’ publicly available information and press releases which it believes to be reliable; however, such reliability can not be guaranteed. Investors should not rely on the information contained on this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, Smallcaps Daily and its owners, affiliates, subsidiaries, officers, directors, representatives, and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of material facts from such advertisement. Smallcaps Daily is not responsible for any claims made by the companies advertised herein, nor is Smallcaps Daily responsible for any other promotional firm, its program, or its structure. Smallcaps Daily is not affiliated with any exchange, electronic quotation system, the Securities Exchange Commission, or FINRA.
OKYO Pharma Limited is a client of TraDigital IR, an investor relations and communications firm. Please see TraDigital’s disclosures at www.tradigitalir.com.
Copyright © 2023 Smallcaps Daily. All rights reserved.