With an Estimated $16.3 Million Market Cap, This Specialty Pharmaceutical Company is Jumping on the pharmaceutical Cannabinoid Opportunity and is Quickly Rising to the Top of its Industry Sector!
SciSparc Ltd. (NASDAQ: SPRC) is aiming to become a leader in the pharmaceutical industry with a robust IP portfolio focused on Pharmaceutical compositions and methods for their use in preventing and treating nervous disorders of the central nervous system.
Download Research Report
Rare cannabinoids are disrupting the pharmaceutical world; early studies have shown the exciting efficacy of cannabinoids against a large variety of diseases.
For example, preclinical and clinical studies have suggested a potential value for CBD in some neuropsychiatric disorders, including epilepsy, anxiety, and schizophrenia!
Cannabinoids are climbing to the top of the market due to it being inexpensive and natural for the user. Cannabinoids are said to produce many medical benefits without the hefty price tag and scary effects of some other types of traditional medicine.
SciSparc Ltd. (NASDAQ: SPRC) is a specialty clinical-stage pharmaceutical company led by an experienced team of senior executives and scientists. The company’s focus is on creating and enhancing a portfolio of technologies and assets based on cannabinoid pharmaceuticals.
The global cannabis pharmaceuticals market size is expected to reach $127.1 billion by 2028!
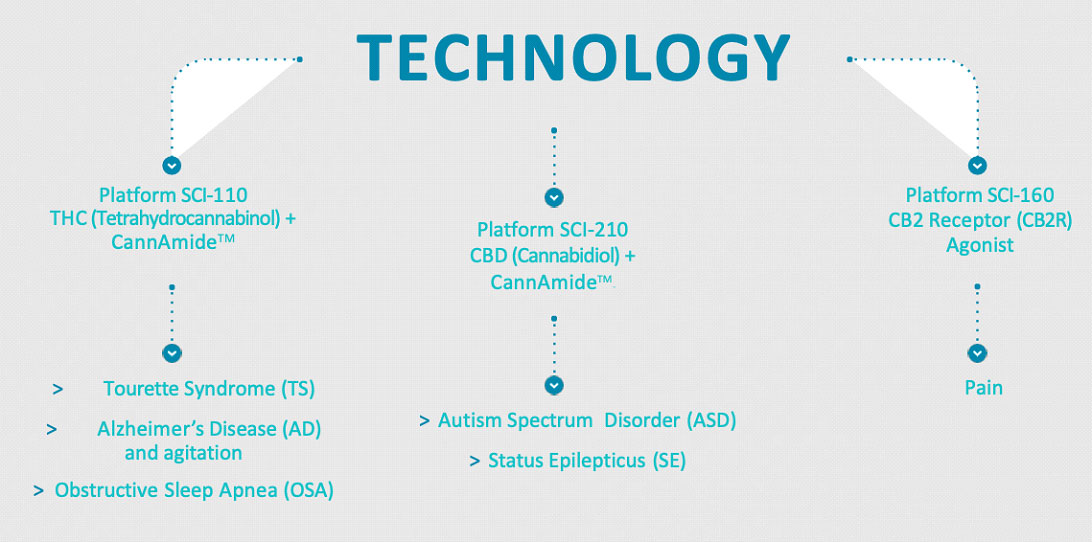
The company is currently engaged in the following drug development programs based on THC and/or non-psychoactive cannabidiol (CBD):
- SCI-110 for the treatment of Tourette syndrome and for the treatment of Alzheimer's disease and agitation.
- SCI-160 for the treatment of pain; and SCI-210 for the treatment of autism spectrum disorder and status epilepticus.
Most recently, SPRC announced completion of the previously announced joint venture agreement to focus on the discovery and development of potential drugs for cancers and other life-threatening conditions, MitoCareX Bio Ltd., an Israeli corporation.
The company also recently appointed Professor Avi Schroeder as a member of the Company's Scientific Advisory Board. Prof. Schroeder will support the development of the Company's top-tier, proprietary, investigational drug candidate SCI-160 program for the treatment of pain.

SPRC is also delving into the world of psychedelics, which have recently been in the spotlight for their healing abilities in relation to mental health.
The company recently entered into a collaboration agreement with Clearmind Medicine Inc. (CSE: CMND), (OTC: CMNDF), (FSE: CWY0) to explore the potential for the creation of innovative compounds in the psychedelic area.
"We believe that the integration of the companies' technologies may lead to a real change in the mental health pharmaceutical market. I'm excited about collaborating with Clearmind as it is a company dedicated to developing novel psychedelic-derived therapeutics to solve widespread and underserved health problems with a strong patent portfolio."
Oz Alder, CEO
Psychedelics have become an increasingly popular area for medical research and studies. A report by Market Digits estimates that the sector could be worth more than $10 billion by 2027, up from $4.75 billion in 2020!
The company is in multiple exciting collaborations/joint ventures in many up-and-coming areas that proves they have undeniable potential for great market growth!
SPRC’s current candidates are designed to treat Tourette Syndrome, Autism Spectrum Disorder, Epilepsy, Obstructive Sleep Apnea, Alzheimer’s Disease and Agitation, and Pain using cannabinoid pharmaceuticals.
The therapeutic potential of using cannabinoids is limitless and are relatively new and unexplored as many cannabis-based products were mostly unavailable before 2018, making companies like SPRC trail-blazers in a very exciting, up and coming market!
The world is on the verge of the cannabinoid-pharmaceutical boom as the study of the application of these molecules has the potential to replace at least part of the opioid market which has had devastating consequences on society economically as well as socially.
- The company has a robust IP portfolio all focused on disorders of the central nervous system using cannabinoid-pharmaceuticals. This put the company in a multi-billion dollar market: The global cannabis pharmaceuticals market size is expected to reach USD 127.1 billion by 2028! The market is expected to expand at a CAGR of 104.2% from 2022 to 2028.
- SPRC recently entered into a collaboration agreement with Clearmind Medicine Inc. (CSE: CMND), (OTC: CMNDF), (FSE: CWY0) to explore the potential for the creation of innovative compounds in the psychedelic area. This puts the company in another favorable market as a report by Market Digits estimates that the sector could be worth more than $10 billion by 2027, up from $4.75 billion in 2020!
- The company’s recent leadership changes positions them for the best possible growth. SPRC is managed by an established team with a proven track record of execution in drug development, working in concert with a world class, award-winning, scientific advisory board chaired by the renowned Professor Raphael Mechoulam. Together they bring decades of experience in biochemistry and pharmacology, research and development, manufacturing, international distribution, clinical and regulatory affairs, as well as financial acumen to the SPRC operation.
- While the usage of cannabis has been popular in recent years, it’s only very recently that we are discovering the rare cannabinoid potential. Early studies have shown the exciting efficacy of cannabinoids against a large variety of diseases. For example, preclinical and clinical studies have suggested a potential value for CBD in some neuropsychiatric disorders, including epilepsy, anxiety, and schizophrenia. Cannabinoids are inexpensive, as well as natural, which is an exciting contrast to traditional forms of drugs and medicine which often have adverse or addictive consequences.
Greetings Investors,
Since the legalization of cannabis, many have found therapeutic, healing qualities of recreational usage of the plant.
This gained so much traction that many medical studies were hot on the trails and began to discover the healing properties for a multitude of ailments! It is more recent, however, that we are discovering the exciting potential of rare, cannabinoid compounds.

Chances are you’ve heard of CBD and THC; the most well-known cannabinoids found in the cannabis plant. However, in the medical world, CBD and THC are just the tip of the iceberg in terms of exciting discovery.
There are over 200 known cannabinoids, including rare cannabinoids like cannabigerol (CBG), cannabinol (CBN), tetrahydrocannabivarin (THCV), and more!
These rare cannabinoids are even more potent in their medical benefits than CBD and don’t contain the psychoactive properties of THC.
These rarer cannabinoids have the potential to completely disrupt the current pharmaceutical market. Cannabinoids are natural, inexpensive, and have proven efficiency against many ailments and diseases such as: sleep disorders, nervous system disorders, neurological, and some neuropsychiatric disorders such as anxiety and epilepsy.
Cannabinoids are different and exciting as they interact with many neurotransmitter and neuromodulator systems in the human body, offering the potential to meet currently unmet needs of patients in multiple treatment areas!
Some of these areas that SPRC are dedicated to include: pain and inflammation, Tourette syndrome, Alzheimer’s disease, Autism Spectrum Disorder, and Status Epilepticus.
The market potential for these areas is HUGE as they are often left untreated, unmanaged, and incurable.
SPRC is playing its part in driving the market high for these areas to help patients find the relief, attention, and treatment they deserve.
Beyond the exciting medical potential, the market for non-traditional forms of medicine such as cannabinoids, cannabis, and psychedelics are growing to become some of the most exciting markets to date.
A report by Market Digits estimates that the psychedelic drug sector could be worth more than $10 billion by 2027, up from $4.75 billion in 2020!
Not only that, but the cannabinoid-pharmaceutical market is alone estimated to be worth $11.2 billion by 2030!
These exciting discoveries puts SciSparc Ltd. (NASDAQ: SPRC) in the best position for growth as it targets massive industries such as cannabinoid-pharmaceuticals!
ABOUT THE COMPANY
Tel Aviv-Based, SciSparc Ltd. (NASDAQ: SPRC) is revolutionizing cannabinoid-based treatments by developing proprietary pharmaceuticals that increase patients’ safety by reducing doses while maintaining effectiveness.
SPRC is managed by an established team with a proven track record of execution in drug development, working in concert with a world class, award-winning, scientific advisory board chaired by the renowned Professor Raphael Mechoulam. Together they bring decades of experience in biochemistry and pharmacology, research and development, manufacturing, international distribution, clinical and regulatory affairs, as well as financial acumen to the SciSparc operation.
SciSparc’s proprietary compounds capitalize on the biochemistry of receptors that specialize in modulating the central nervous system to create therapeutics that mitigate the adverse symptoms associated with CNS disorders.
COMPANY HIGHLIGHTS
- The company’s primary platforms focus on the endocannabinoid system. The SciSparc pipeline composes both FDA – approved, and New Chemical Entities (NCEs) which they believe have strong potential to be indicated for targeted conditions, with no existing or sufficiently effective therapies.
- Enhancing existing compounds with their proprietary technologies, SPRC has developed novel therapies that demonstrate in clinical trials heightened bioavailability, significantly improved efficacy, lower dosage requirements, better safety profiles and a reduction in side effects.
- The company has four drug candidates in clinical trials with applications for Tourette Syndrome, Alzheimer’s Disease and Agitation, Autism Spectrum Disorder, and two in pre-clinical studies for Status Epilepticus, and Chronic Pain.
- The company’s research and development efforts are focused on creating a proprietary and unique combination of products, some available already in the market (CannAmide™), products in the accelerated regulatory path of 505 (b)(2) application (SCI-110, SCI-210) and products that constitute a new chemical entity (SCI-160) in order to bring different products to market at different timelines and potentially enable them to generate immediate revenues for the company, augmenting FDA-approved natural and synthetic cannabinoids in combination with our proprietary compounds and technologies to create alternate therapies that potentiate the effects of cannabinoids and target the receptors implicated in modulating the central nervous system.
ROBUST IP PORTFOLIO
Repurposing previously approved compounds qualifies SPRC to take advantage of the FDA’s 505 (b)(2) application protocol. This potentially means lower risk, and an expedited development timeline. The result is a robust pipeline of product candidates well positioned to create significant shareholder value into the future.
The company has built an intellectual property portfolio which currently comprises three granted U.S. patents and pending patent applications in six families, all focused on disorders of the central nervous system. Currently, their drug candidates are designed to treat Tourette Syndrome, Autism Spectrum Disorder, Epilepsy, Obstructive Sleep Apnea, Alzheimer’s Disease and Agitation, and Pain.
PRODUCTS
The company’s proprietary drug candidate, containing Dronabinol, FDA approved synthetic form of THC, with the endocannabinoid palmitoylethanolamide (PEA).
Designed to stimulate cannabinoid receptors across the Central Nervous System and inhibit the metabolic degradation of endocannabinoids in order to improve uptake of THC, the expected benefits of SCI-110 are an increase in efficiency of oral administration, and in turn a decrease in dosage requirements, side effects and adverse events.
This product is being developed under the accelerated regulatory path of 505 (b)(2) application focused on augmenting FDA-approved natural and synthetic cannabinoids to create alternate therapies that potentiate the effects of cannabinoids and target the receptors implicated in modulating the central nervous system.
This approach qualifies us for access to the FDA’s 505 (b)(2) regulatory strategy, created to facilitate the submission of novel drug candidates that meet specific criteria to the FDA for review. The 505 (b)(2) application provides us with several advantages as compared to a typical New Drug Application, including potential; lower risk and development costs, and a potentially expedited time to market.
Indications currently being investigated for treatment with SCI-110 include:
– Tourette Syndrome- (TS)
– Obstructive Sleep Apnea (OSA)
– Alzheimer’s Disease and Agitation
SCI-110 for TOURETTE SYNDROME (TS) PHASE IIA Led by Yale University
TS is a movement and neurobehavioral disorder characterized by motor and vocal tics and is highly linked with co-morbidities.
As the currently used medications are managing only a small number of disease symptoms with limited efficacy and questionable safety, there is a clear unmet medical need for the management of TS
Results from their Phase IIA clinical trial conducted in Yale University:
An average tic reduction of 21% across the entire sample with almost 40% of the patients experiencing greater than 25% in tic reduction as defined by YGTSS-TTS (a clinician-rated instrument considered as the gold standard for assessing tics in patients with Tourette's Syndrome)
The medication was generally well-tolerated by subjects
12/16 subjects elected to continue into a 24-week extension phase of the trial.
SCI-110 for TS PHASE IIB
- Objective: to evaluate the efficacy, safety and tolerability of the Company’s proprietary SCI-110 in a randomized, double-blind, placebo controlled, cross- over study
- Two medical centers: Hannover Medical School, Hannover, Germany and Tel- Aviv Sourasky Medical Center, Tel-Aviv, Israel
- 1:1 ratio randomization to receive either SCI-110 or SCI-110 matched placebo (i.e., THC active, CannAmideTM placebo)
- Design: 12 weeks treatment, washout period of 2 weeks, crossover for another 12 weeks
- Primary efficacy: change in YGTSS-R-TTS1 as a continuous endpoint at week 12 and week 26 of the double-blind phase compared to baseline
- Primary safety: absolute and relative frequencies of Serious Adverse Events (SAEs) for the whole population and separately for SCI-110 and placebo groups
SCI-110 for ALZHEIMER’S DISEASE (AD) & AGITATION Phase IIA
AD or mixed dementia (AD + Vascular Dementia) accounts for over 2/3 of all dementia(s)* with majority of AD patients' manifest agitation and anxiety.
Objective: to evaluate the safety, tolerability and efficacy trend of SCI-110 in an open label study in patients with Alzheimer Disease (AD) and Agitation Clinical site: Sophie & Abraham Stuchynski Israeli Alzheimer's Medical Center Israel
20 patients will be treated with SCI-110 in a daily dose of up to 12.5mg THC+800 mg PEA
Primary end point: safety and tolerability of SCI -110 in AD patients with agitation
Main efficacy trend: The ability SCI -110 to ameliorate agitation in patients with AD as measured by the Cohen Mansfield Agitation Inventory (CMAI).
SPRC’s proprietary drug candidate containing cannabidiol (CBD), a non-psychoactive cannabinoid, and PEA.
This product is initially being developed under the regulation of the Israeli Medical Cannabis Agency (IMCA) – the agency that leads the regularization of the medical cannabis field in Israel and is the first of its kind in the world. It is a complex, unique, innovative and original process. Conducting clinical trials and development under the regulation of the HQR ostensibly enables rapid and specific registration processes in a track that is unique to Israel.
The company intends to further develop the product for markets outside Israel as well. Indications currently being investigated for treatment with SCI-210 include:
- Autism Spectrum Disorder (in clinical trials)
- Status Epilepticus – a form of seizures that are severe and sometimes fatal. This indication is currently investigated in pre-clinical settings.
SCI-210 FOR AUTISM SPECTRUM DISORDER (ASD) Phase III*
ASD is a condition related to brain development that impacts how a person perceives and socializes with others, causing problems in social interaction and communication.
Study objective: to evaluate the safety, tolerability and efficacy of SCI-210 in children with ASD in a randomized, double- blind, placebo controlled with cross-over study.
Study design: A 20-week, randomized double-blind placebo-controlled with cross-over clinical trial of 60 children, 30 participants for SCI-210, or SCI-210 placebo (CBD active, CannAmideTM placebo) for 20 weeks, Washout for 2 weeks, Crossed over for additional 20 weeks
Three primary efficacy end points: The Aberrant Behavior Checklist-Community (ABC-C) parent questionnaire; The Clinical Global Impressions-Improvement (CGI-I) performed by a clinician.
SCI-210 for STATUS EPILEPTICUS (SE)
Status Epilepticus is a seizure that lasts longer than 5 minutes or one in which a person has more than 1 seizure within a 5-minute period, without returning to a normal level of consciousness between episodes.
There is limited pharmaceutical treatment available for treating epilepsy, as about one in three patients have drug- resistance against epilepsy!
In June 2018, the FDA approved purified plant-derived CBD (Epidiolex) for the treatment of seizures associated with Lennox-Gastaut and Dravet syndromes in patients aged two years and older. But CBD monotherapy has several adverse effects, the most common being lethargy, elevated liver enzymes, decreased appetite, diarrhea, poor sleep quality and infections.
In addition, CBD therapy requires a constant increase in therapeutic doses to stay effective and leads to the development of tolerance.
The Company intends to utilize its proprietary SCI-210 platform, combining PEA with CBD treatment to achieve maximum effect with minimal adverse side effects in Epilepsy patients. PEA Significantly Increases CBD Effects**: CBD was shown to prevent fat accumulation in liver cells in a concentration dependent manner .PEA by itself didn’t affect fat accumulation. Addition of PEA to CBD reduced the required CBD concentration while achieving maximal effect.
An innovative, proprietary synthetic CB2 receptor agonist created, among others, for the treatment of pain and is currently in pre-clinical studies.
The CB2 receptor agonist used in this formulation – HU-433 – was invented and synthesized by Professor Raphael Mechoulam, Ph.D., Chairman of the SciSparc Scientific Advisory Board, and is protected under a patent granted in the U.S. and Europe.
SCI-160 FOR PAIN
An innovative and proprietary CB2 Receptor (CB2R) agonist formulation intended for the treatment of pain. This specific CB2R agonist was synthesized by Professor Raphael Mechoulam, Ph.D.
CB2 Receptor specific agonists have been found to be involved in mediating analgesic effects in the peripheral nervous system, without significant side effects.

After successful completion of pre-clinical studies, the Company will file an FDA Investigational New Drug (IND) Phase I study application.
SCI-160
PRE-CLINICAL RESULTS
In a Von Frey Test, rats were evaluated for tactile allodynia using a Von Frey Filament. Promising results demonstrate analgesic 40 efficacy is better than morphine.
An immediate unique palmitoylethanolamide (PEA) oral formulation for the reduction of chronic pain and inflammation. PEA is a cannabinoid mimetic lipid molecule found throughout the body, including the central nervous system. Similar to cannabinoids, PEA has been shown to have neuroprotective, anti-inflammatory, analgesic and anti-convulsant properties.


The legalization of cannabis in recent years, as well and the rise in awareness among consumers regarding health benefits of cannabis and its growing medical application such as treatment of wide range of diseases and symptoms, including:
Cancer, chronic pain, depression, arthritis, diabetes, glaucoma, migraines, epilepsy, multiple sclerosis, acquired immunodeficiency syndrome (AIDS), amyotrophic lateral sclerosis (ALS), Alzheimer’s, post-traumatic stress disorder (PTSD), Parkinson’s, and Tourette’s have been driving the market for medical cannabis to impressive growth!
This is a multi-billion dollar market! The global cannabis pharmaceuticals market size is expected to reach USD 127.1 billion by 2028! The market is expected to expand at a CAGR of 104.2% from 2022 to 2028.
Furthermore, the global cannabis market size is projected to reach $176 Billion by 2030 with the legalization for medicinal purposes leading this growth
MARKET POTENTIAL
- The global Alzheimer’s therapeutics market is projected to reach $13.68 by 2027 from $7.48 in 2019.
- The global epilepsy market size would grow from $8.8B in 2018 to $9.5B towards the end of 2023
- The global ASD therapeutics market was approximately $3.3B in 2018 and is expected to reach approximately $4.6B by 2026
- Tourette Syndrome (TS) market was $80M in 2019 and is expected to reach $98.7M by 2023
- The global chronic pain treatment market was valued at $77.8B in 2019
The Company Team
Oz Adler - CEO, CFO
Mr. Adler has experience in a wide variety of managerial, financial, tax and accounting practices. From 2012 to 2017, Mr. Adler was employed as a certified public accountant at Kost Forer Gabbay & Kasierer, a member of Ernst & Young Global. Mr. Adler holds a B.A. degree in Accounting and Business management from the College of Management, Israel.
Adi Zuloff- Shani, PhD – CTO
Dr. Zuloff- Shani is a Research & Development professional with overall experience of about 20 years in the bio-tech and healthcare industry. Dr.Zuloff-Shani has brought two products from bench to market; an immuno-cell-based product and a food-supplement and is currently leading the development of several pharmaceutical products designated to the U.S., EU, and Israeli markets. Dr. Zuloff-Shani has extensive experience in research and development, manufacturing, clinical, and regulatory affairs. Dr. Zuloff-Shani holds a Ph.D. in human biology and immunology from Bar-Ilan University, Israel.
Amitay Weiss- Chairman
Mr. Weiss serves as chairman of the board of directors of P.L.T Financial Services Ltd., as chairman of the board of directors of Matomy Media Group Ltd. and as an external director of Cofix Group Ltd. In 2016, Mr. Weiss founded Amitay Weiss Management Ltd. and now serves as its chief executive officer.
Itschak Shrem- President & Director
Mr. Shrem has more than 40 years of experience in financial markets and venture capital. In 1991, Mr. Shrem founded Dovrat Shrem Ltd., an investment banking, management and technology company. Prior to that, he spent 15 years at Clal Israel Ltd., where he served in various capacities, including chief operating officer, and was responsible for capital markets and insurance businesses. In 1993, Mr. Shrem founded Pitango Venture Capital Fund (formerly, Polaris) and served as a partner of Pitango Funds I, II and III. He has been the Managing Director of Yaad Consulting 1995 Ltd. since 1995. Mr. Shrem currently serves on the board of directors of Rail Visions Ltd. Previously, Mr. Shrem served on the board of Tel-Aviv Sourasky Medical Center, the Weizmann Institute Eden Spring Ltd., Nano Dimension Ltd., Ormat Industries Ltd., Retalix Ltd. and as chairman of Sphera Funds Management Ltd. Mr. Shrem holds a B.A. in Economics and Accounting from Bar-Ilan University and an M.B.A. from Tel-Aviv University.
THE BOTTOM LINE
Tel Aviv-Based, SciSparc Ltd. (NASDAQ: SPRC) is revolutionizing cannabinoid-based treatments by developing proprietary pharmaceuticals that increase patients’ safety by reducing doses while maintaining effectiveness.
The company’s robust IP portfolio focused on disorders of the central nervous system, puts the company in very exciting markets that have seen recent growth.
SPRC is dedicated to developing unique cannabinoid technologies for treatments. The global cannabis pharmaceuticals market size is expected to reach USD 127.1 billion by 2028!
The company’s strong leadership team and recent joint venture with Clearmind Medicine Inc. puts the company in the world of psychedelics, which have recently been in the spotlight for their healing abilities in relation to mental health.
A report by Market Digits estimates that the psychedelic sector could be worth more than $10 billion by 2027, up from $4.75 billion in 2020!
With an estimated $16.3 million market cap and sitting in exciting markets, SPRC should be at the top of your watch-list as it is positioned for impressive growth!
Hurry and Start Your Research Now!

THIS IS A PAID ADVERTISEMENT
NO INVESTMENT ADVICE
This Website Is Wholly Owned By Tradigital Marketing Group, Inc. (D/B/A “Tradigital Ir”). Our Reports Are Advertorials And Are For General Information Purposes Only. Never Invest In Any Stock Featured On Our Site Or Emails Unless You Can Afford To Lose Your Entire Investment. The Disclaimer Is To Be Read And Fully Understood Before Using Our Services, Joining Our Email List, As Well As Any Social Networking Platforms We May Use. Please Note Well: Tradigital Ir And Its Employees Are Not Registered Investment Advisors, Broker-Dealers, Or Member(S) Of Any Association For Other Research Providers In Any Jurisdiction Whatsoever. Release Of Liability: Through Use Of This Website, Viewing Or Using You Agree To Hold Tradigital Ir, Its Operators, Owners, And Employees Harmless And To Completely Release Them From Any And All Liability Due To Any And All Loss (Monetary Or Otherwise), Damage (Monetary Or Otherwise), Or Injury (Monetary Or Otherwise) That You May Incur. The Information Contained Herein Is Based On Sources That We Believe To Be Reliable But Is Not Guaranteed By Us As Being Accurate And Does Not Purport To Be A Complete Statement Or Summary Of The Available Data. Tradigital Ir Encourages Readers And Investors To Supplement The Information In These Reports With Independent Research And Other Professional Advice. All Information On Featured Companies Is Provided By The Companies Profiled Or Is Available From Public Sources And Tradigital Ir Makes No Representations, Warranties, Or Guarantees As To The Accuracy Or Completeness Of The Disclosure By The Profiled Companies. None Of The Materials Or Advertisements Herein Constitute Offers Or Solicitations To Purchase Or Sell Securities Of The Companies Profiled Herein And Any Decision To Invest In Any Such Company Or Other Financial Decisions Should Not Be Made Based Upon The Information Provided Herein. Instead, Tradigital Ir Strongly Urges You To Conduct A Complete And Independent Investigation Of The Respective Companies And Consideration Of All Pertinent Risks. Tradigital Ir’s Full Disclosure Is To Be Read And Fully Understood Before Using Tradigital Ir's Website, Or Joining Tradigital Ir's Email Or Text List. From Time To Time, Tradigital Ir Will Disseminate Information About A Company Via Website, Email, Sms, And Other Points Of Media. By Viewing Tradigital Ir's Website And/Or Reading Tradigital Ir's Email Or Text Newsletter You Are Agreeing ----> Https://Tradigitalir.Com/